- EN - English
- CN - 中文
Isolation of Immunocomplexes from Zebrafish Brain
斑马鱼脑中免疫复合物的分离
发布: 2023年04月05日第13卷第7期 DOI: 10.21769/BioProtoc.4646 浏览次数: 1389
评审: Xi FengQin TangAnonymous reviewer(s)
Abstract
Zebrafish is an excellent model to study vertebrate neurobiology, but its synaptic components that mediate and regulate fast electrical synaptic transmission are largely unidentified. Here, we describe methods to solubilize and immunoprecipitate adult zebrafish brain homogenate under conditions to preserve electrical synapse protein complexes. The methods presented are well-suited to probe electrical synapse immunocomplexes, and potentially other brain-derived immunocomplexes, for candidate interactors from zebrafish brain.
Keywords: Immunoprecipitation (IP) (免疫沉淀(IP))Background
Immunoprecipitation (IP) and, under favorable conditions, co-immunoprecipitation (co-IP) can be used to isolate proteins and associated complexes in vivo (Harlow and Lane, 1999). Unlike column affinity chromatography that requires large amounts of starting material, the immunoprecipitation technique enriches target proteins and possibly their associated complexes from a small amount of material. The challenge of successfully isolating immunocomplexes from in vivo tissue is identifying an antibody that specifically and reproducibly binds the target protein, while also co-precipitating directly and indirectly interacting proteins. While polyclonal and monoclonal antibodies each have their own benefits, with the former recognizing multiple epitopes of a target and the latter recognizing a single epitope, it is not possible to predict success a priori. Therefore, it is crucial to test a variety of antibodies to find one that reproducibly immunoprecipitates and co-immunoprecipitates associated complexes. Additional steps critical to successful isolation of immunocomplexes include identifying a detergent that solubilizes the tissue for optimal release of the target protein and maintaining cold temperatures throughout the isolation to prevent protein degradation. Here, we present a protocol optimized to detect electrical synapse immunocomplexes from zebrafish brain (Lasseigne et al., 2021; see flow chart in Figure 1). The method is useful to identify protein–protein interactions in vivo and, though not validated for other tissues, could be adapted to other zebrafish organs and protein complexes, making this a helpful biochemical tool for the zebrafish community.
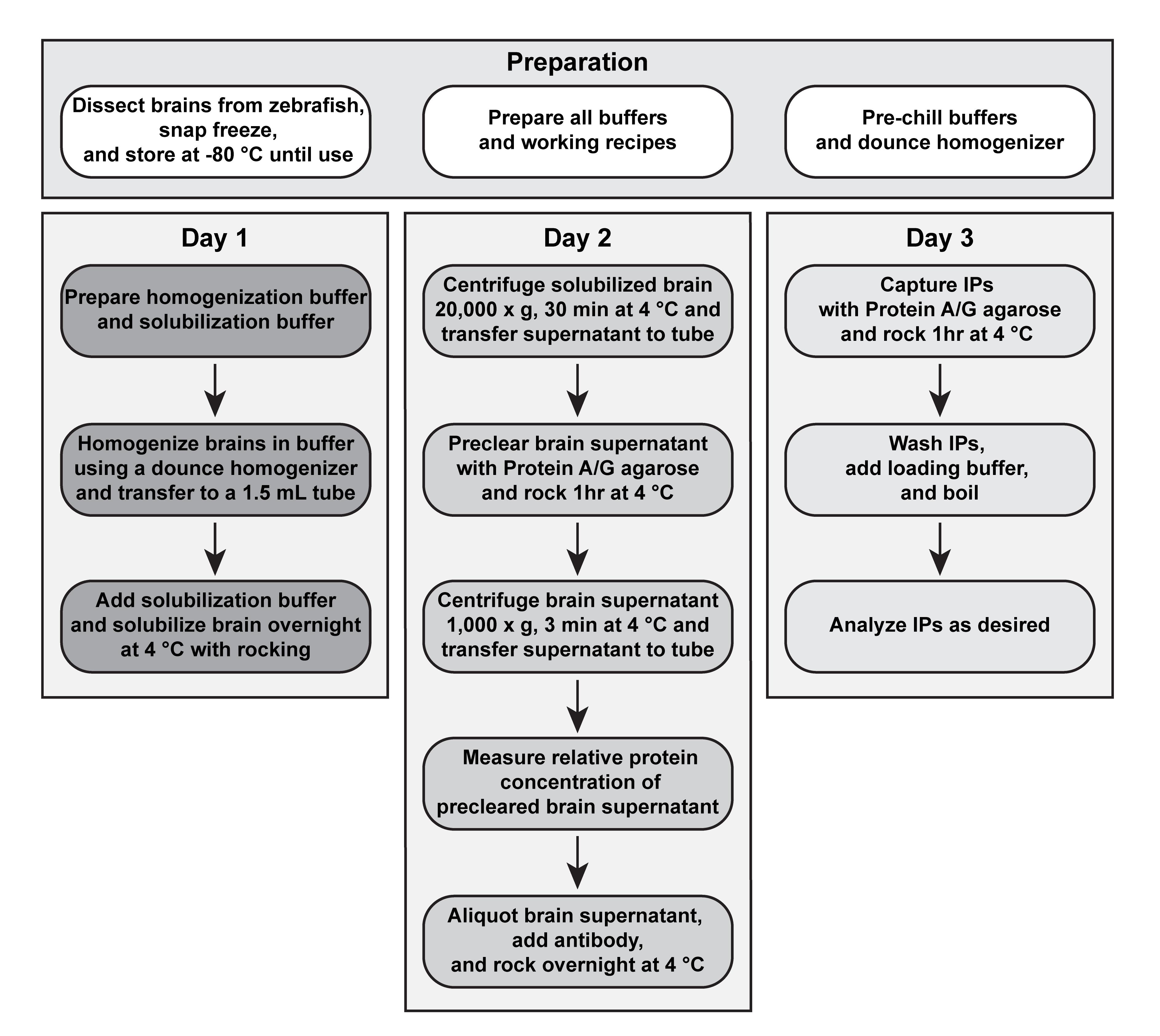
Figure 1. Flowchart for isolation of immunocomplexes from zebrafish brain
Materials and Reagents
1.5 mL plastic cuvettes (Fisher, catalog number: 14-955-127)
1.5 mL Eppendorf Snap-Cap Safe-Lock microcentrifuge tubes (Fisher, catalog number: 05-402-25)
0.65 mL graduated microtube (Fisher, catalog number: NC9260727)
Zebrafish brains (from fish ages 3 months–1.5 years) snap frozen in liquid nitrogen and stored at -80 °C
Antibody [This will vary with each experiment. We have successfully used anti-ZO1 monoclonal antibody (ZO1-1A12) (ThermoFisher, catalog number: 33-9100) for co-immunoprecipitation.]
n-Octyl-β-D-Glucopyranoside (Anatrace, catalog number: O311 5 GM)
Pierce protease inhibitor mini tablets, EDTA-free (Fisher, catalog number: PIA32955)
Dithiothreitol (DTT) reducing agent (Bio-Rad, catalog number: 610611)
HEPES (Fisher, catalog number BP310-500)
NaCl (Fisher, catalog number: BP358-212)
EDTA, disodium salt dihydrate (Fisher, catalog number: BP120-1)
EGTA (Sigma, catalog number: E-4378)
NaOH pellets (Fisher, catalog number: M1064621000)
Pierce Protein A/G agarose (Fisher, catalog number: PI20421)
4× Laemmli sample buffer (Bio-Rad, catalog number: 1610747)
Bio-Rad Protein Assay dye reagent concentrate (Bio-Rad, catalog number: 5000006)
1 M HEPES pH 7.5 (see Recipes)
5 M NaCl (see Recipes)
0.5 M EDTA pH 8.0 (see Recipes)
0.5 M EGTA pH 8.0 (see Recipes)
1 M DTT (see Recipes)
Homogenization buffer (see Recipes)
Solubilization buffer (see Recipes)
Preclear buffer (see Recipes)
IP wash buffer (see Recipes)
2× loading buffer (see Recipes)
Note: See Recipes to create working solutions.
Equipment
1 mL dounce tissue grinder with loose and tight pestles (Fisher, catalog number: 50-365-300 or similar)
Benchtop tube rocker (Benchmark Scientific M2100 or similar)
Refrigerated centrifuge (Eppendorf 5430 R or similar)
Spectrophotometer (Eppendorf BioPhotometer or similar)
Procedure
Tissue preparation—Freeze and store brains
Note: Adult fish should be euthanized according to proper guidelines for the ethical care and use of animals in research associated with IACUC protocols of the user’s institution.
Euthanize an adult fish and dissect the brain out as described in Gupta and Mullins (2010; see detailed video at: https://www.jove.com/t/1717).
Transfer each dissected brain to an Eppendorf tube, immediately close the cap tightly, and snap freeze tissue by immersing the tube in liquid nitrogen for at least one minute.
Note: Liquid nitrogen handling should follow appropriate lab safety guidelines.
When all brains are dissected, transfer snap frozen brains to -80 °C freezer until use.
Note: Individual brains are stored separately and combined below dependent upon experimental needs. Frozen brains can be stored at -80 °C for at least two years.
DAY ONE—Homogenize brains
Note: The following protocol describes conditions for two IPs. It is important to maintain all reagents on ice throughout the procedure to prevent protein degradation.
Freshly prepare 5 mL of homogenization buffer by adding one protease inhibitor mini tablet and 5 µL of 1 M DTT; keep on ice.
Note: Homogenization buffer is prepared in bulk and is sufficient for 50 brains. Use within 60 min of preparation to ensure optimal protease inhibitor activity and DTT stability during tissue homogenization.
Freshly prepare 5 mL of solubilization buffer by adding 5 µL of 1 M DTT; keep on ice.
Note: Solubilization buffer is prepared in bulk and is sufficient for 50 brains. Use within 60 min of preparation to ensure DTT stability during tissue solubilization.
Put five frozen zebrafish brains into an ice-cold 1 mL dounce tissue grinder.
Note: The resulting quantity of total protein from each brain will vary depending on the age and size of the adult fish dissected. As a general guideline, using two brains per IP often results in 1–2 mg total protein/IP, though the total quantity required will vary according to protein expression levels and antibody efficiency.
Add 0.5 mL of prepared ice-cold homogenization buffer.
Note: Save unused homogenization buffer at 4 °C for use on DAY TWO, step C10 to mix the blank solution needed for the protein assay.
Homogenize brain tissue with 10–20 strokes using the loose pestle and then 10–20 strokes using the tight pestle.
Note: Homogenizing in the absence of detergent prevents oxidation of proteins. Also, if needed, glass homogenizers and pestles can be decontaminated between samples by rinsing extensively with distilled water, then 70% ethanol, and then distilled water again before drying with air.
Transfer the homogenate (0.5 mL) to a 1.5 mL Eppendorf tube using a 1,000 µL pipette tip.
Add 0.5 mL of prepared solubilization buffer and gently invert to thoroughly mix the solutions.
Note: The prepared solubilization buffer contains 4% detergent, so that when mixed with an equal volume of brain homogenate, the final detergent concentration is 2%. Save unused solubilization buffer at 4 °C for use on DAY TWO, step C10 to mix the blank solution needed for the protein assay.
Solubilize the brain tissue by rocking the tube on a benchtop rocker at 24 rpm, overnight at 4 °C.
DAY TWO—Preclear and set up immunoprecipitations
Prepare pre-washed Pierce Protein A/G agarose by washing a 250 µL bed volume with 1 mL of preclear buffer.
Note: Pre-washed Pierce Protein A/G agarose is prepared in bulk and is sufficient for three preparations.
Spin at 1,000 × g for 3 min at 4 °C.
Remove buffer and repeat wash steps C1 and C2 two more times using new preclear buffer each time.
After the final wash, remove buffer and resuspend agarose in 250 µL of preclear buffer to achieve a 500 µL 50% slurry of pre-washed Pierce Protein A/G agarose. Store pre-washed agarose at 4 °C until use in step C7 below and in DAY THREE, step D2.
Next, centrifuge the overnight solubilized brain homogenate at 20,000 × g for 30 min at 4 °C.
Without disturbing the pellet, transfer the solubilized brain supernatant to a new 1.5 mL Eppendorf tube using a 1,000 µL pipette tip.
Preclear the brain supernatant by adding 100 µL of 50% slurry of pre-washed Pierce Protein A/G agarose (prepared in steps C1–C4), and rock on benchtop rocker at 24 rpm for 1 h at 4 °C.
Spin the supernatant at 1,000 × g for 3 min at 4 °C to pellet the agarose.
Without disturbing the agarose, carefully transfer the precleared supernatant to a new 1.5 mL Eppendorf tube using a 1,000 µL pipette tip.
Measure the relative quantity of total protein using Bio-Rad protein assay according to the manufacturer’s instructions (see detailed protocol at https://www.bio-rad.com/webroot/web/pdf/lsr/literature/LIT33.pdf; 1.5 mL plastic cuvettes and a spectrophotometer are required).
Note: For the blank solution, mix equal quantities of homogenization buffer and solubilization buffer that were saved at 4 °C from DAY ONE, steps B4 and B7.
Save an extract sample by transferring 40 µL of precleared brain supernatant to a new 0.65 mL microtube and adding 40 µL of 2× loading buffer. Save at -20 °C until needed for SDS-PAGE electrophoresis on DAY THREE, step D12.
To set up IPs, transfer 400 µL of precleared brain supernatant to each of two new 1.5 mL Eppendorf tubes (this is approximately 1–2 mg total protein/IP).
Note: If comparing IPs from different samples, be sure to immunoprecipitate an equal quantity of total protein in an equal volume. The minimum volume for an IP is 200 µL. Also, use the precleared extract for immunoprecipitation immediately. Do not freeze the precleared supernatant or the protein complexes will fall apart, and co-IP of associated proteins will fail.
Add 2 µg of antibody per IP.
Note: Anywhere from 0.5–5 µg can be used, depending on the efficiency of the antibody.
Rock the IPs on a benchtop rocker at 24 rpm, overnight at 4 °C.
DAY THREE—Capture immunoprecipitates
Centrifuge the IPs at 1,000 × g for 1 min at 4 °C.
Add 30 µL of 50% slurry of pre-washed Pierce Protein A/G agarose (prepared in DAY TWO steps C1–C4 and stored at 4 °C) to each IP.
Rock IPs on a benchtop rocker at 24 rpm for 1 h at 4 °C.
Centrifuge IPs at 1,000 × g for 3 min at 4 °C.
Remove supernatant with a 1,000 µL pipette tip without disturbing the agarose.
Add 1 mL of IP wash buffer to the agarose and invert several times.
Spin 1,000 × g for 3 min at 4 °C.
Repeat wash steps D5–D7 two more times, using new IP wash buffer each time.
On the last wash, completely remove the wash buffer from the agarose using a 200 µL pipette tip, followed by a flattened 10 µL gel loading tip.
Add 20 µL of 2× loading buffer.
Boil extract sample and immunoprecipitations for 3 min at 95 °C.
Note: Boiling may cause aggregation of membrane proteins. In this case, alternative options include omitting step D11 or heating the samples at 65 °C for 10 min instead. Best results need to be empirically determined per experiment.
Continue with protein analysis as desired, e.g., SDS-PAGE and western blot (Towbin et al., 1979; Sambrook and Russell, 2006; Ni et al., 2017).
Note: The subsequent protein analysis steps are dependent upon the equipment available. For a comprehensive guide, see https://www.bio-rad.com/webroot/web/pdf/lsr/literature/Bulletin_6040.pdf.
Recipes
1 M HEPES, pH 7.5 (500 mL)
Dissolve 119.15 g of HEPES (MW 238.5 g/mol) in 400 mL of ultrapure water. Adjust pH to 7.5 with 10 N NaOH. Adjust final volume to 500 mL with ultrapure water.
5 M NaCl (500 mL)
Dissolve 146 g of NaCl (MW 58.44) in 400 mL of ultrapure water. Adjust final volume to 500 mL with ultrapure water.
0.5 M EDTA, pH 8.0 (100 mL)
Dissolve 18.61 g of EDTA, disodium salt dihydrate (MW 372.24 g/mol) in 80 mL of ultrapure water. Adjust the pH to 8.0 with NaOH pellets. Adjust the final volume to 100 mL with ultrapure water.
0.5 M EGTA, pH 8.0 (100 mL)
Dissolve 19.02 g of EGTA (MW 380.35 g/mol) in 80 mL of ultrapure water. Adjust the pH to 8.0 with 4 N NaOH. Adjust final volume to 100 mL with ultrapure water.
1 M DTT (10 mL)
Dissolve 1.5 g of DTT in 8 mL of ultrapure water. Adjust volume to 10 mL, dispense into 1 mL aliquots, and store at -20 °C.
Homogenization buffer (50 mL)
20 mM HEPES pH 7.5 1.0 mL 1 M HEPES pH 7.5
150 mM NaCl 1.5 mL 5 M NaCl
5 mM EDTA 0.5 mL 0.5 M EDTA
5 mM EGTA 0.5 mL 0.5 M EGTA
46.5 mL ultrapure water
Solubilization buffer (25 mL)
20 mM HEPES pH 7.5 0.5 mL 1 M HEPES pH 7.5
150 mM NaCl 0.75 mL 5 M NaCl
5 mM EDTA 0.25 mL 0.5 M EDTA
5 mM EGTA 0.25 mL 0.5 M EGTA
4% n-Octyl-β-D-Glucopyranoside 1 g n-Octyl-β-D-Glucopyranoside
Adjust final volume to 25 mL with ultrapure water
Preclear buffer (10 mL)
20 mM HEPES pH 7.5 5 mL homogenization buffer
150 mM NaCl 5 mL solubilization buffer
5 mM EDTA
5 mM EGTA
2% n-Octyl-β-D-Glucopyranoside
IP wash buffer (25 mL)
20 mM HEPES pH 7.5 23.75 mL homogenization buffer
150 mM NaCl 1.25 mL solubilization buffer
5 mM EDTA
5 mM EGTA
0.2% n-Octyl-β-D-Glucopyranoside
2× loading buffer (1 mL)
2× Laemmli sample buffer 0.5 mL 4× Laemmli sample buffer
200 mM DTT 0.2 mL 1 M DTT
0.3 mL ultrapure water
Acknowledgments
This protocol was used in the companion paper Lasseigne et al. (2021) Elife (DOI: 10.7554/eLife.66989). National Institute of Mental Health (RF1MH120016) to Alberto Pereda and Adam C. Miller. National Institute of Neurological Disorders and Stroke (R01NS105758) to Adam C. Miller.
Competing interests
We declare no competing interests.
Ethics
Studies using this protocol were performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Animals were handled according to approved institutional animal care and use committee (IACUC) protocols (#AUP 21-42) of the University of Oregon.
References
Gupta, T. and Mullins, M. C. (2010). Dissection of organs from the adult zebrafish. J Vis Exp(37): 1717.
- Harlow, E. and Lane, D. (1999). Using antibodies: a laboratory manual. N.Y.: Cold Spring Harbor Laboratory Press.
- Lasseigne, A. M., Echeverry, F. A., Ijaz, S., Michel, J. C., Martin, E. A., Marsh, A. J., Trujillo, E., Marsden, K. C., Pereda, A. E. and Miller, A. C. (2021). Electrical synaptic transmission requires a postsynaptic scaffolding protein. Elife 10: e66898.
- Ni, D., Xu, P. and Gallagher, S. (2017). Immunoblotting and Immunodetection. Curr Protoc Protein Sci 88: 10.10.1-10.10.37.
- Sambrook, J. and Russell, D. W. (2006). SDS-Polyacrylamide Gel Electrophoresis of Proteins. CSH Protoc 2006(4): pdb.prot4540.
- Towbin, H., Staehelin, T. and Gordon, J. (1979). Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications.Proc Natl Acad Sci U S A 76: 4350-4354.
文章信息
版权信息
Michel and Miller. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Michel, J. C. and Miller, A. C. (2023). Isolation of Immunocomplexes from Zebrafish Brain. Bio-protocol 13(7): e4646. DOI: 10.21769/BioProtoc.4646.
- Lasseigne, A. M., Echeverry, F. A., Ijaz, S., Michel, J. C., Martin, E. A., Marsh, A. J., Trujillo, E., Marsden, K. C., Pereda, A. E. and Miller, A. C. (2021). Electrical synaptic transmission requires a postsynaptic scaffolding protein. Elife 10: e66898.
分类
神经科学 > 细胞机理 > 蛋白质分离
生物化学 > 蛋白质 > 分离和纯化
分子生物学 > 蛋白质 > 蛋白质-蛋白质相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













