- EN - English
- CN - 中文
Live Imaging of Phagoptosis in ex vivo Drosophila Testis
体外果蝇睾丸中吞噬作用的实时成像
发布: 2023年03月20日第13卷第6期 DOI: 10.21769/BioProtoc.4637 浏览次数: 1836
评审: Gal HaimovichRafael Sênos DemarcoRajesh D GunageAnonymous reviewer(s)
Abstract
Phagoptosis is a prevalent type of programmed cell death (PCD) in adult tissues in which phagocytes non-autonomously eliminate viable cells. Therefore, phagoptosis can only be studied in the context of the entire tissue that includes both the phagocyte executors and the targeted cells doomed to die. Here, we describe an ex vivo live imaging protocol of Drosophila testis to study the dynamics of phagoptosis of germ cell progenitors that are spontaneously removed by neighboring cyst cells. Using this approach, we followed the pattern of exogenous fluorophores with endogenously expressed fluorescent proteins and revealed the sequence of events in germ cell phagoptosis. Although optimized for Drosophila testis, this easy-to-use protocol can be adapted to a wide variety of organisms, tissues, and probes, thus providing a reliable and simple means to study phagoptosis.
Background
Several types of programmed cell death (PCD) have been described, all of which function to eliminate excess or damaged cells throughout development and to maintain tissue homeostasis in adult organisms (Fuchs and Steller, 2011). Phagoptosis, a newly recognized type of PCD, is a form of cell non-autonomous cannibalism in which one cell uses the phagocytic machinery to induce death and degradation of a nearby cell that would otherwise remain alive [(Brown and Neher, 2012); Figure 1A]. Accordingly, inhibiting the phagocytic machinery within phagocytes prevents the death of targeted cells. In adults, phagoptosis is the most prevalent form of PCD, mediating the turnover of several abundant short-lived cells, including erythrocytes (Olsson and Oldenborg, 2008) and neutrophils (Jitkaew et al., 2009). This important process was only recently recognized because it can occur only in the context of entire tissues that include the two cell types, i.e., phagocytes and targeted live cells. Therefore, developing assays and finding biomarkers for this process represents a major step in characterizing the process and identifying the regulators of phagoptosis.

Figure 1. Phagoptosis of germ cell progenitors. A. The mechanism of phagoptosis. A phagocyte (pink) engulfs a live-targeted cell (green), creating a phagosome around that target, and recruits the phagocytic machinery (Rab5, Rab7, Lamp1, and lysosomes) to degrade its contents. B. Schematic representation of the apical tip of the Drosophila testis (side view). Germline (green) and cyst (pink) stem cells surround the hub cells (gray). Approximately a quarter of the spermatogonia progenitors (green) undergo spontaneous phagoptosis (red) by neighboring phagocytic cyst cells (pink). Dashed line separates progenitors from terminally differentiated spermatocytes.
In the Drosophila testis, as many as a quarter of newly emerging spermatogonia progenitors are spontaneously eliminated by germ cell death (GCD) ( Yacobi-Sharon et al., 2013; Napoletano et al., 2017). We recently showed that the underlying mechanism of GCD is phagoptosis (Zohar-Fux et al., 2022). The cyst cells that surround the germ cell progenitors rely on the phagocytic machinery to induce death and degradation of targeted germ cells (Figure 1B).
Several fluorescent markers can be used to mark the phagocytic cyst cells during the live imaging protocol, including cytoplasmic green fluorescent protein (cytGFP; Figure 2 and Video 1) or membrane-targeted CD8-GFP (Lee and Luo, 1999)]. To follow the activity of the cyst cell–derived phagocytic machinery, we used yellow fluorescent protein (YFP) tags inserted at the DNA level at the endogenous chromosomal loci of Rab5- and Rab7-containing endosomes [Video 2 (Dunst et al., 2015)]. During live imaging, we maintain the testes in a medium containing a low concentration of LysoTracker and Hoechst to visualize in situ changes in lysosomal activity and DNA integrity, respectively. Intriguingly, this technique revealed the formation of large blebs pinching off the dying germ cells that were not detected in fixed samples. While the surfaces of these blebs were positive for LysoTracker, their inner content was negative for such staining, suggesting that nutrients of the dying germ cells are transported to the cyst cells via these blebs [Video 2 (Zohar-Fux et al., 2022)]. In this protocol, we dissect whole-mount intact testes and visualize the spontaneously occurring phagoptosis process for 4–12 hours. Since the testis is a highly regenerative tissue and the germline stem and progenitor cells divide frequently, the entire tissue throbs, which may affect imaging resolution. Therefore, tight adherence of the testis to the chamber without damaging its integrity is the most critical step in obtaining a detailed live imaging movie. A previous live imaging protocol designed to study the stem cell niche of the Drosophila testis suggested the use of the isotonic Ringer's solution as the dissection medium (Greenspan and Matunis, 2017). Indeed, using Ringer's solution significantly improves the ability of the light testis to sink to the bottom of the coverslip and facilitates the adherence process.
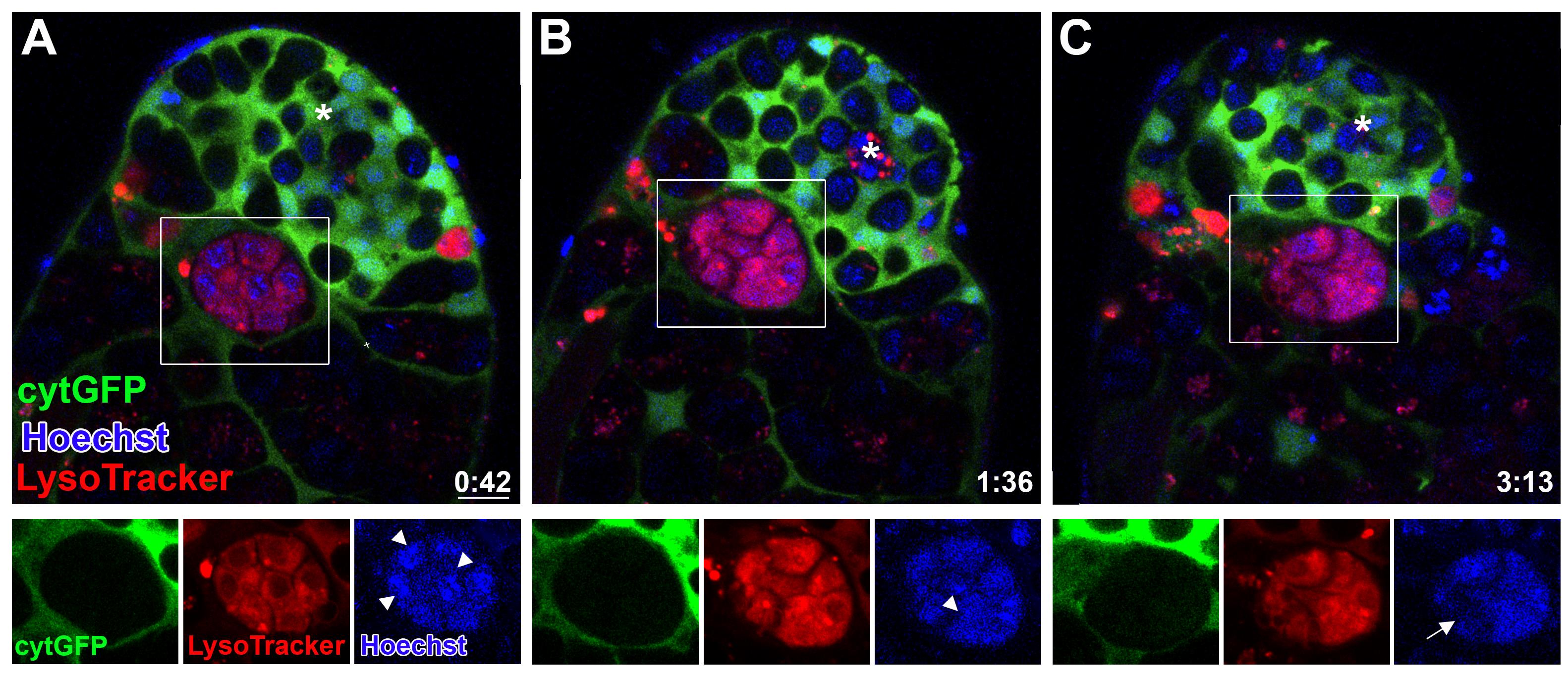
Figure 2. Phagoptosis of germ cell progenitors. A-C. Snapshots of live-imaged testis, marked with LysoTracker (red), Hoechst (blue, nuclei), and GFP (cyst cells, c587Gal4;UAS-cytGFP). Time (hour:min) is shown on the bottom right corner. Note the rectangles and bottom images (single-channel views of the boxed regions) highlighting a phagoptosis event. White arrowheads mark DNA packed into separate nuclei (A and B) that are further involuted into one bundle (C, arrow). Asterisks mark the hub, and scale bars correspond to 10 μm.
In developing this protocol, we tested several adherence methods, including the use of 0.1% gelatin, Concanavalin A (Con A, 100 ng/mL), double layers of Con A and fetal calf serum (FCS; 10%), and two concentrations of Poly-L-Lysine (0.01% and 0.1%). We found that the dissected testes could be adhered only on Poly-L-Lysine and that the higher concentration of Poly-L-Lysine (0.1%) enabled easy adherence without applying excessive force that could damage the tissue. Coating the dish with poly-L-lysine for immobilization was shown to be detrimental to the development of early germ line cysts (Gartner et al., 2014).
Materials and Reagents
Eppendorf tubes
10 µL pipettor
Poly L-Lysine solution, 0.1% (w/v) in H2O (Sigma-Aldrich, catalog number: P8920)
Fetal bovine serum (Biological Industries, catalog number: 04-007-1A)
Penicillin/streptomycin (Biological Industries, catalog number: 03-031-1B)
Schneider’s Drosophila medium (Biological Industries, catalog number: 01-150-1A)
Hoechst stain (Invitrogen, catalog number: H3570)
LysoTracker (Thermo Fisher Scientific, catalog number: L7528)
Immersion oil (Nikon, catalog number: M210009219)
Dulbecco's phosphate buffered saline (PBS, 10×) (Biological Industries, catalog number: 02-023-5A)
Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9888-500G)
Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P3911-500G)
Calcium chloride (CaCl2) (Sigma-Aldrich, catalog number: C1016-500G)
Magnesium chloride (MgCl2) (Sigma-Aldrich, catalog number: M8266-100G)
Sucrose (Sigma-Aldrich, catalog number: S0389-500G)
HEPES solution (1 M, pH 6.9) (Sigma-Aldrich, catalog number: H0887-20ML)
Schneider’s medium (see Recipes)
Ringer’s solution (see Recipes)
Equipment
Standard fly husbandry equipment (e.g., vials with freshly prepared standard cornmeal molasses agar medium, 25 °C incubators, CO2 and fly pad station to anaesthetize flies, and brushes to sort and transfer the flies)
Chambered coverslip containing eight wells (ibidi, catalog number: 80826; Figure 3A)
Superfrost microscope slides (Thermo Scientific, catalog number: 631-0705). A superfrost slide is used as a dissecting dish and can be re-used after cleaning with 70% ethanol.
Dumont #5 blunter forceps (e.g., Fine Science Tools, catalog number: 11252-20)
Dumont #55 sharper forceps (e.g., Fine Science Tools, catalog number: 11255-20)
Zooming stereomicroscope with 5 × 40 magnification (e.g., Carl Zeiss, model: STEMI SV 11)
Confocal microscope. (e.g., Nikon A1R inverted laser scanning confocal microscope with a Plan Apochromat VC 60× oil objective)
Biological safety cabinet (hood) (e.g., ESCO, model: AC2-4S1)
Software
Microscope controller software (e.g., Nikon NIS Elements C v. 4.2 software, https://www.microscope.healthcare.nikon.com/products/software/nis-elements)
Cell imaging software (e.g., Imaris for Cell Biologists, Oxford Instruments, https://imaris.oxinst.com/)
ImageJ/FIJI https://imagej.net/downloads
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Kanaan, D., Shklyar, B., Porat-Kuperstein, L. and Toledano, H. (2023). Live Imaging of Phagoptosis in ex vivo Drosophila Testis. Bio-protocol 13(6): e4637. DOI: 10.21769/BioProtoc.4637.
- Zohar-Fux, M., Ben-Hamo-Arad, A., Arad, T., Volin, M., Shklyar, B., Hakim-Mishnaevski, K., Porat-Kuperstein, L., Kurant, E. and Toledano, H. (2022). The phagocytic cyst cells in Drosophila testis eliminate germ cell progenitors via phagoptosis. Sci Adv 8(24): eabm4937.
分类
细胞生物学 > 细胞成像 > 共聚焦显微镜
发育生物学 > 繁殖
细胞生物学 > 组织分析 > 组织成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













