- EN - English
- CN - 中文
Automated Sleep Deprivation Setup Using a Shaking Platform in Mice
在小鼠中使用振动平台自动睡眠剥夺设置
发布: 2023年02月20日第13卷第4期 DOI: 10.21769/BioProtoc.4620 浏览次数: 2060
评审: Christine Muheim Shaorong MaAnonymous reviewer(s)
Abstract
The functions of sleep remain largely unclear, and even less is known about its role in development. A general strategy to tackle these questions is to disrupt sleep and measure the outcomes. However, some existing sleep deprivation methods may not be suitable for studying the effects of chronic sleep disruption, due to their lack of effectiveness and/or robustness, substantial stress caused by the deprivation method, or consuming a large quantity of time and manpower. More problems may be encountered when applying these existing protocols to young, developing animals, because of their likely heightened vulnerability to stressors, and difficulties in precisely monitoring sleep at young ages. Here, we report a protocol of automated sleep disruption in mice using a commercially available, shaking platform–based deprivation system. We show that this protocol effectively and robustly deprives both non-rapid-eye-movement (NREM) sleep and rapid-eye-movement (REM) sleep without causing a significant stress response, and does not require human supervision. This protocol uses adolescent mice, but the method also works with adult mice.
Graphical abstract

Automated sleep deprivation system. The platform of the deprivation chamber was programmed to shake in a given frequency and intensity to keep the animal awake while its brain and muscle activities were continuously monitored by electroencephalography and electromyography.
Background
The functions of sleep and its long-term impact on behavior and physiology have been largely understudied. Studies focusing on the acute effect of sleep deprivation (SD) in juvenile mice, rats, and cats revealed that sleep affects synaptic structure and plasticity during adolescent development (Frank et al., 2001; Dumoulin Bridi et al., 2015; Shaffery et al., 2006; Shaffery et al., 2002; Maret et al., 2011; Yang et al., 2014; W. Li et al., 2017; de Vivo et al., 2017). However, whether these changes result in long-lasting behavioral changes remains unknown. To evaluate the impact of long-term sleep perturbation, especially during development, chronic sleep disruption is required, and so is longitudinal sleep monitoring using electroencephalogram (EEG) along the manipulation process.
Automated SD in rodents often uses physical stimulations or setups that keep the animal in constant motion, such as using continuously moving treadmills or rotating wheels (Colavito et al., 2013). In an alternating platform setup, the animal was forced to move between two small platforms that continuously and alternatively move above and below water (Pierard et al., 2007). In a setup of spinning disk above a water tank, the disk rotated whenever sleep onset was detected, and the animal would need to stay awake and mobile in order not to fall into the water (they woke up in the case they did fall) (Rechtschaffen et al., 1983). Another method is the so-called “grid over water,” where placing the animal on a grid floor suspended over the water surface significantly reduced NREM and REM sleep and increased sleep latency, but the reduction of sleep was only in half (Shinomiya et al., 2003). REM-selective deprivation can be achieved by placing the animal on a small platform surrounded by and slightly above the water surface (Morden et al., 1967). The animal was allowed to have NREM sleep by crouching on the platform; however, upon transition to a REM episode, the loss of muscle tone caused the animal to touch or fall into the water and thus aroused the animal (Morden et al., 1967). These automated SD paradigms all produce stress, anxiety, and/or excessive physical stimuli, making them unsuitable for adolescent mice, which likely have heightened vulnerability to stressors (Romeo, 2013). In comparison, more mild methods such as the “gentle touch” protocol requires constant monitoring of the mouse behavior or EEG by an experimenter, as whenever the mouse exhibits signs of sleep (e.g., staying still for longer than 5 s, or low-frequency EEG), the experimenter directly intervenes by touching the animal using a soft tool (e.g., a brush), keeping the animal awake (Eban-Rothschild et al., 2016; W. Li et al., 2017; Yang et al., 2014). However, this method is labor-intensive, and may not be easily replicated between experimenters; thus, it is not ideal if high throughput or chronic intervention is desired. Another “gentle” protocol requires exposing the animal to novel objects, a different environment, and/or nesting materials, which leads to exploratory or nest-building behaviors, respectively, and helps maintain wakefulness. This method does not cause significant stress (Kopp et al., 2006), but the animal tends to become habituated quickly, and the forced wakefulness usually does not last longer than 2 h. In addition, pre-exposure to the enrichment materials may be a confounder, if a behavior task such as the novel object recognition test is performed subsequently.
Here, we report an alternative protocol for automated SD in mice, which is effective for 4 h or longer and robust across many days, does not cause significant stress even in adolescent animals, and can be performed in high throughput (up to four mice at a time for each chamber) without human intervention. The protocol is ideal for chronic sleep disruptions over days, in both developing and adult mice. In combination with a closed loop algorithm, it is also possible to achieve REM-selective automated deprivation.
Materials and Reagents
Paper towels
Male or female mice with a C57BL/6J background produced by breeding pairs purchased from Jackson Laboratory, or with a 129S2/SvPasCrl background produced by breeding pairs purchased from Charles River. They were born and housed at constant temperature (22 ± 1 °C) and humidity (40%–60%), under a 12/12 h light:dark cycle [lights-on: 7:00–19:00, zeitgeber time (ZT) 0–12; lights-off: 19:00–7:00, ZT 12–24], with access to food and water ad libitum. Animals were single-housed in customized home-cages after implantation of the electrodes for EEG and electromyography (EMG) (see Equipment).
(Optional) An ultra-light extension cord (~50 cm in length, Cooner wires, catalog number: CW8183) was used for reducing the cable weight on adolescent mice during EEG/EMG recording; however, this is not required for adult mice.
Corticosterone ELISA kit (Enzo, catalog number: ADI-900-097, storage temperature: 4 °C)
Microvette® CB 300 Lithium heparin (Sarstedt Inc., catalog number: 16.443.100)
Hydrogel (ClearH2O, catalog number: 70-01-5022)
70% ethanol
Equipment
Sleep Deprivation System (ViewPoint Life Sciences, Inc., Lyon, France), composed of a deprivation chamber (PVC cylinder, height: 46 cm, width: 30 cm, weight: 5 Kg) with a shaking platform at the bottom, a control box, and a computer with the controlling program Shaker Driver (Figure 1A, B). A video from the vendor illustrating the setup and its use can be found in https://youtu.be/LNTc7c5yFZM.
A customized home-cage (Figure 1C) was made by taping two standard mouse cages together (without the lid, dimensions, 28 cm × 17.5 cm × 12.5 cm). A big opening (approximately 22 × 11 cm) was made on the top for the extension cord/recording cable to pass through, and a small hole was drilled on the side of bottom part, for water access. Regular mouse food pellets were placed inside the cage together with the bedding.
Eppendorf centrifuge 5424 (Eppendorf, Hamburg, Germany).
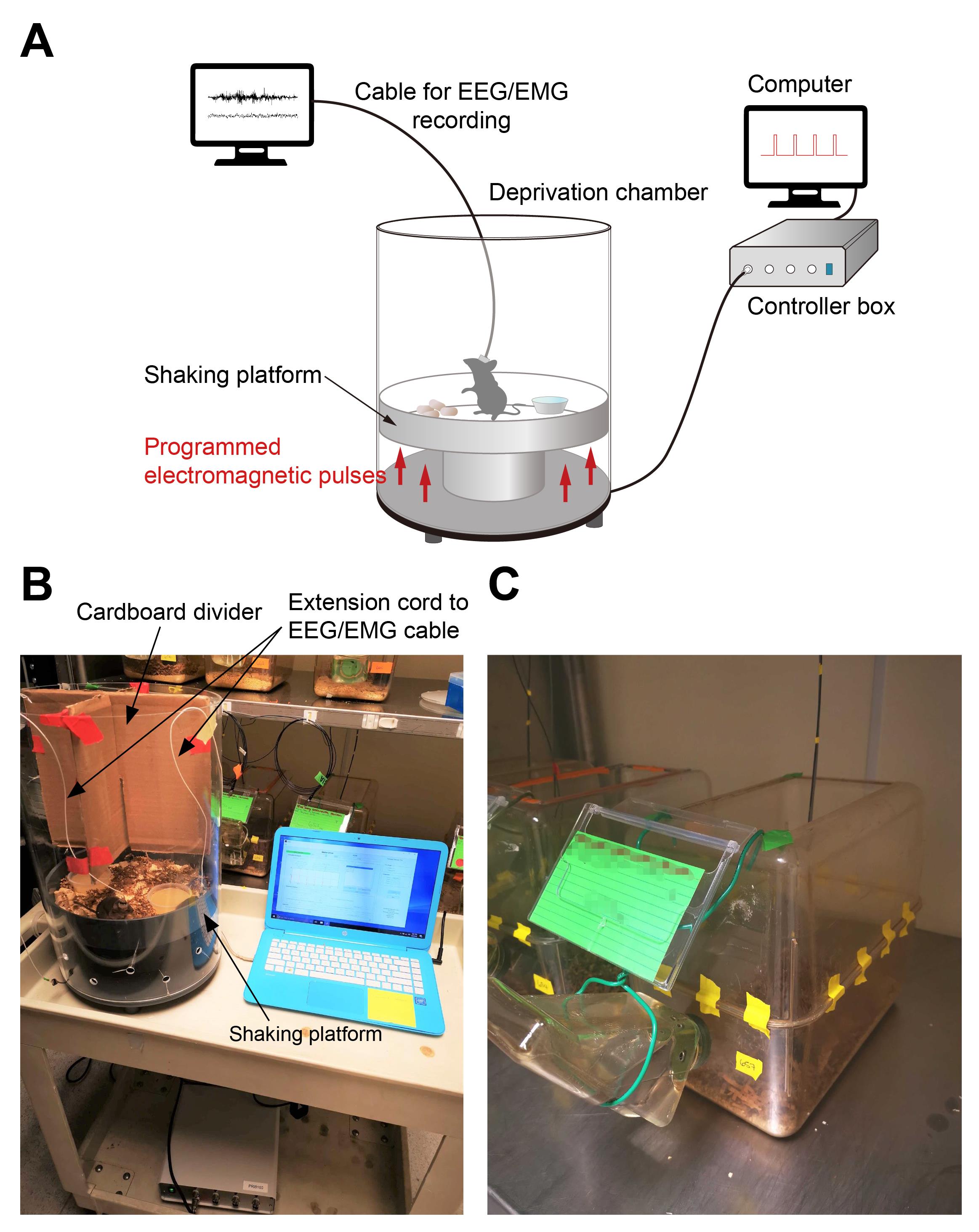
Figure 1. Automated sleep deprivation system and the customized home-cage. A. Schematics of the automated sleep deprivation system with simultaneous EEG/EMG recording. B. Sleep deprivation chamber. C. Customized home-cage.
Software
Shaker Driver v1.9.4 (ViewPoint Life Sciences) (Figure 2)
Standard data processing and statistics software: Excel, Prism (GraphPad; version 8 or higher), etc.
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Bian, W. J. and de Lecea, L. (2023). Automated Sleep Deprivation Setup Using a Shaking Platform in Mice . Bio-protocol 13(4): e4620. DOI: 10.21769/BioProtoc.4620.
分类
神经科学 > 行为神经科学 > 睡眠与觉醒
神经科学 > 发育
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link














