- EN - English
- CN - 中文
Isolation and Culture of Primary Fibroblasts from Neonatal Murine Hearts to Study Cardiac Fibrosis
从新生小鼠心脏中分离和培养原代成纤维细胞以研究心脏纤维化
发布: 2023年02月20日第13卷第4期 DOI: 10.21769/BioProtoc.4616 浏览次数: 3836
评审: Giusy TornilloMohan BabuThirupugal Govindarajan

相关实验方案
用于比较人冷冻保存 PBMC 与全血中 JAK/STAT 信号通路的双磷酸化 CyTOF 流程
Ilyssa E. Ramos [...] James M. Cherry
2025年11月20日 2282 阅读
Abstract
Cardiac fibroblasts are one of the major constituents of a healthy heart. Cultured cardiac fibroblasts are a crucial resource for conducting studies on cardiac fibrosis. The existing methods for culturing cardiac fibroblasts involve complicated steps and require special reagents and instruments. The major problems faced with primary cardiac fibroblast culture are the low yield and viability of the cultured cells and contamination with other heart cell types, including cardiomyocytes, endothelial cells, and immune cells. Numerous parameters, including the quality of the reagents used for the culture, conditions maintained during digestion of the cardiac tissue, composition of the digestion mixture used, and age of the pups used for culture determine the yield and purity of the cultured cardiac fibroblasts. The present study describes a detailed and simplified protocol to isolate and culture primary cardiac fibroblasts from neonatal murine pups. We demonstrate the transdifferentiation of fibroblasts into myofibroblasts through transforming growth factor (TGF)-β1 treatment, representing the changes in fibroblasts during cardiac fibrosis. These cells can be used to study the various aspects of cardiac fibrosis, inflammation, fibroblast proliferation, and growth.
Keywords: Cardiac fibroblasts (心肌成纤维细胞)Background
Cardiac fibroblasts or fibrocytes are one of several cells constituting a healthy heart. These are non-myocyte cells involved in developing and maintaining the cardiac architecture (Snider et al., 2009). In a physiological condition, they interact with other heart cells and maintain cardiac homeostasis (Kurose, 2021). Cardiac fibroblasts preserve the structural integrity of the heart by producing and depositing collagen I, III, V, and VI and fibronectin as part of the extracellular matrix (ECM) (Snider et al., 2009). Fibroblasts are the major players in modulating ECM by degrading its proteins through the secretion of matrix metalloproteinases (Eghbali and Weber, 1990; Chacar et al., 2017).
In a healthy heart, one of the largest populations of cells is the fibroblasts (Camelliti et al., 2005). During cardiac stress or injury, fibroblasts divide and differentiate into myofibroblasts or activated fibroblasts with an increase in their activity (Rohr, 2011). To compensate for the loss of cardiomyocytes during a pathophysiological condition, activated fibroblasts deposit ECM proteins, such as collagen, to sustain the structural integrity of the heart (Kurose, 2021). However, if deposited in excess, the ECM expands the cardiac interstitium, a characteristic of cardiac fibrosis that leads to heart dysfunction (N. G. Frangogiannis, 2020) and premature death. Since cardiac fibroblasts hold great significance in the functioning of a heart, it is essential to characterize the various roles played by fibroblasts (Rohr, 2011).
To investigate various cellular aspects of cardiac fibroblasts in detail, including changes in cellular morphology, regulatory molecular pathways, gene-expression levels, and changes in the expression level of proteins, cultured neonatal primary cardiac fibroblasts can prove to be a valuable model system. Cultured neonatal cardiac fibroblasts mimic various characteristics of fibroblasts in vitro. These cells have the propensity to phenotypically differentiate into myofibroblasts, thus recapitulating the conditions of a stressed or injured heart (Rohr, 2011). Obtaining pure primary cardiac fibroblasts and procuring homogeneity in the cells is indeed very advantageous. It allows for studies to be carried out on these specific cells without any interference from other heart cell types or other regulatory paracrine signaling pathways that can affect their activity. Cultured cardiac fibroblasts can also be subjected to treatments stimulating these cells to mimic cardiac pathologic conditions, such as cardiac fibrosis, in vitro.
Transforming growth factor β (TGF-β) plays a significant role in fibrogenesis in the fibroblasts (N. Frangogiannis, 2020). There are three isoforms of TGF-β: TGF-β1, TGF-β2, and TGF-β3 (Schiller et al., 2004). All three have been shown to contribute to fibrogenesis. However, TGF-β1 has been reported to be the most important isoform involved in fibrosis (Yu et al., 2003). The signaling pathways involved in driving fibrosis are either Smad3-dependent or Smad3-independent pathways, involving various co-receptors and regulators (N. Frangogiannis, 2020). Primary cardiac fibroblasts can be used to study various signaling pathways involved in cardiac fibrosis, including the TGF- β/SMAD3 signaling pathway. Overall, primary cardiac fibroblasts act as a reliable in vitro model that can be used to induce and mimic fibrosis that takes place in vivo.
Isolation and culture of primary cardiac fibroblasts from neonatal rat and mouse pups
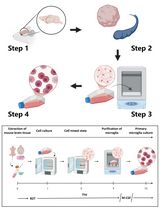
This protocol describes an easy procedure for isolating and culturing cardiac fibroblasts from neonatal rat and mouse pups. The described procedure involves the use of enzymes such as collagenase type II and trypsin for the digestion of the heart tissue (to digest the ECM and dissociate the cells). No additional growth supplements are required for the culture of primary cardiac fibroblasts. Tissue culture plates are coated with poly-L-lysine to enhance the attachment of cardiac fibroblasts. The procedure includes dissecting the rat/mouse pups and taking out the hearts. This is followed by making small chunks of the heart and digesting the heart tissue with the prepared digestion mixture (trypsin + collagenase). Eventually, collection of the digested cells is done in horse serum; the collected cells are plated in tissue culture plates and incubated in an incubator for further subculturing and experimentation. The whole procedure takes approximately 4–5 h (Figure 1).
It is advised for the users to first consult the institutional guidelines on animal usage for experiments and to get the necessary permissions from the institute’s animal ethics committees. Gloves and lab coats should always be worn when handling animals, and there should be no direct contact of bare hands with animals and/or tissues. Carcasses and tissue wastes generated while harvesting the pups should be collected and sealed in double bags and disposed of in an incinerator, following institutional guidelines.
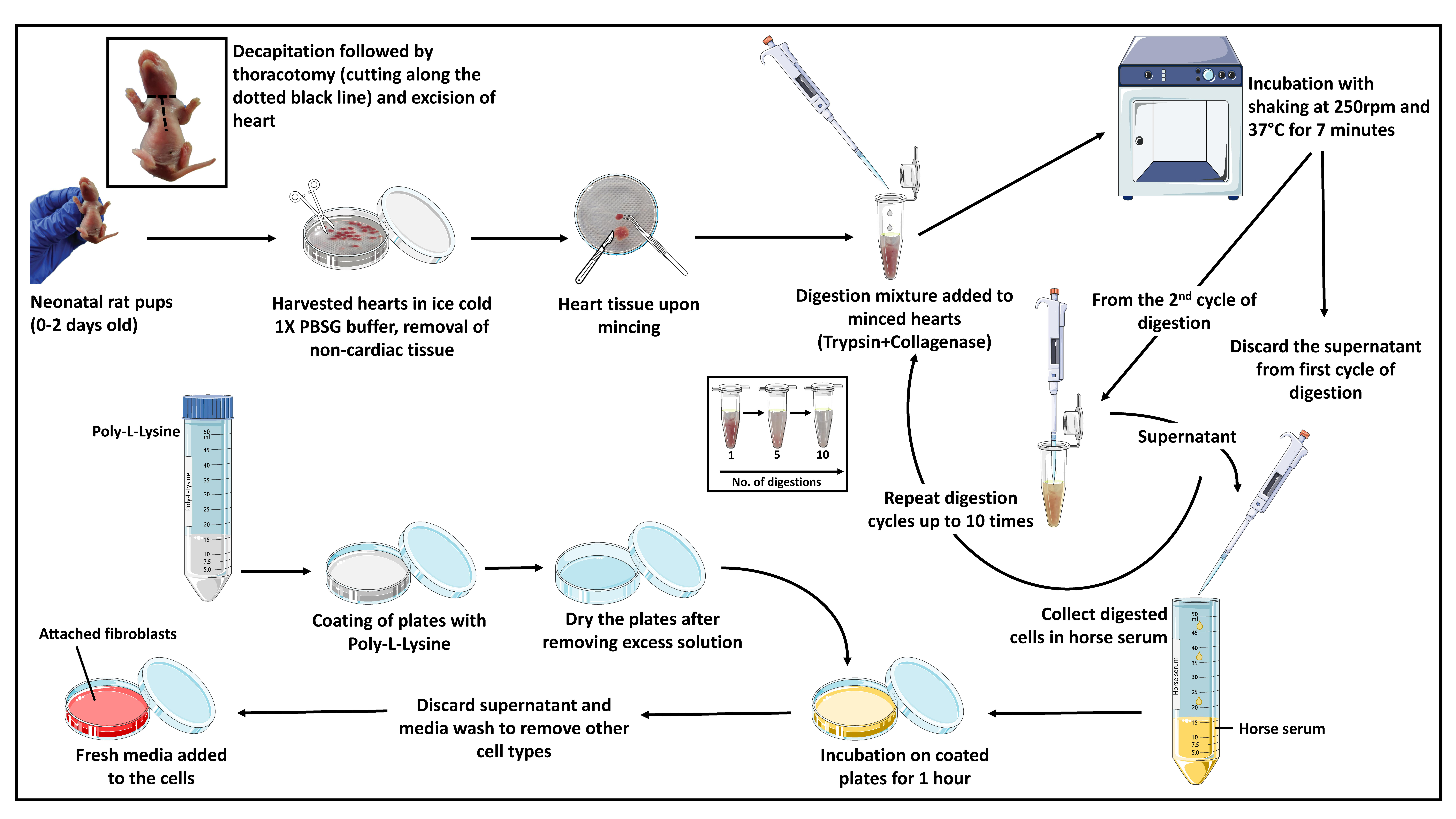
Figure 1. Diagrammatic representation of the major steps involved in the isolation and culture of primary cardiac fibroblasts from murine pup hearts. The figure was designed using images adapted from Servier Medical Art by Servier. Original images are licensed under a Creative Commons Attribution 3.0 Unported License.
Basic protocol: Isolation and culture of primary cardiac fibroblasts from neonatal murine pups
Materials and Reagents
Sterile cotton
Sterile 10 cm Petri plates (Eppendorf, catalog number: EP0030702115)
Sterile 1.5 mL microcentrifuge tubes (Eppendorf, catalog number: 0030121872)
50 mL conical centrifuge tubes (Eppendorf, catalog number: 0030122178)
Rat pups or mouse pups (0–2 days old)
Distilled water
70% ethanol (HIMEDIA, catalog number: MB228) (v/v) in double distilled water
Horse serum (Gibco, catalog number: 16050122)
Trypsin 0.25% solution 1× without phenol red (HIMEDIA, catalog number: TCL006)
Complete media: Dulbecco’s Modified Eagle Medium (DMEM) (HIMEDIA, catalog number: AL007A), supplemented with 10% fetal bovine serum (FBS) (HIMEDIA, catalog number: RM-10432) and 1× antibiotic-antimycotic mix (Gibco, catalog number: 15640-055) (see Recipes)
Phosphate-buffered saline (PBS), 1× (see Recipes)
Phosphate-buffered saline buffer (1×) with D-glucose (PBSG) (see Recipes)
Digestion mixture (DM) (see Recipes)
Equipment
Sterile surgical scissors (Harvard Apparatus, catalog number: ST2 72-8438)
Forceps (Harvard Apparatus, catalog number: ST2 72-8949)
Blades (Harvard Apparatus, catalog number: ST2 72-8366)
Tissue culture laminar hood (vertical flow) (Thermo Scientific, model: 1338)
Vortex mixer (SPINIX by Tarsons, 3020)
Shaker incubator (Thermo, model: MAXQ 4450)
CO2 incubator for cell culture (maintained at 37 °C and 5% CO2)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Kumar, S., Nagesh, D., Ramasubbu, V., Prabhashankar, A. B. and Sundaresan, N. R. (2023). Isolation and Culture of Primary Fibroblasts from Neonatal Murine Hearts to Study Cardiac Fibrosis. Bio-protocol 13(4): e4616. DOI: 10.21769/BioProtoc.4616.
- Maity, S., Muhamed, J., Sarikhani, M., Kumar, S., Ahamed, F., Spurthi, K. M., Ravi, V., Jain, A., Khan, D., Arathi, B. P., et al. (2020). Sirtuin 6 deficiency transcriptionally up-regulates TGF-beta signaling and induces fibrosis in mice. J Biol Chem 295(2): 415-434.
分类
细胞生物学 > 细胞信号传导 > 胞内信号传导
细胞生物学 > 细胞分离和培养 > 细胞分离
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link