- EN - English
- CN - 中文
Imaging of Chloroplast Movement Responses to Light Stimulation in Different Intensities in Rice
水稻不同强度光刺激下叶绿体运动的成像
(*contributed equally to this work) 发布: 2023年02月20日第13卷第4期 DOI: 10.21769/BioProtoc.4611 浏览次数: 1445
评审: Wenrong HeYao XiaoAnonymous reviewer(s)
Abstract
Chloroplast movement has been observed and analyzed since the 19th century. Subsequently, the phenomenon is widely observed in various plant species such as fern, moss, Marchantia polymorpha, and Arabidopsis. However, chloroplast movement in rice is less investigated, presumably due to the thick wax layer on its leaf surface, which reduces light sensitivity to the point that it was previously believed that there was no light-induced movement in rice. In this study, we present a convenient protocol suitable for observing chloroplast movement in rice only by optical microscopy without using special equipment. It will allow researchers to explore other signaling components involved in chloroplast movement in rice.
Keywords: Imaging (成像)Background
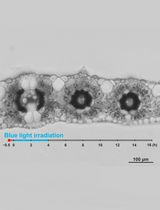
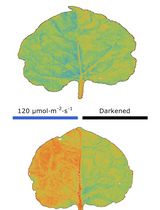
The chloroplast, as a plant-specific photosynthetic organelle, has different responses to different light intensities (Kong and Wada, 2016). Under weak light, chloroplasts accumulate at the front face of the cells to absorb enough light for photosynthesis; under strong light, chloroplasts move to the anticlinal cell wall to avoid photo-damage (Wada, 2013). In model systems such as Arabidopsis thaliana, a number of components and downstream signaling proteins have been shown to mediate chloroplasts movement signaling in response to different light conditions (Kong and Wada, 2014). However, the molecular mechanism of chloroplast movement in rice still remains unclear, due to limitations on experimental methods and detection tools.
The main methods used for detecting chloroplast movement include: (i) green and white band assay, (ii) optical microscope, (iii) measurement of red-light transmittance, (iv) confocal laser scanning microscope, and (v) leaf cross section. Due to the complexity of rice’s leaf structure, the existing methods are not suitable (Suetsugu et al., 2017).
In this protocol, we describe a method to detect chloroplast movement using rice leaf sheath. The method is based on a protocol established for Arabidopsis and rice (Zhang et al., 2022), which we have adapted and optimized for convenient and accurate analysis of rice and other gramineous plants. There is no doubt that the system will be beneficial for further understanding of the molecular mechanisms and evolutionary history of chloroplast movement in green plants.
Materials and Reagents
9 cm Petri dish (Sangon Biotech, catalog number: F611001)
Hydroponic tank (Koraba, catalog number: 53506733434)
Razor blade and forceps
1.5 mL tube (Nantong Haidi Experimental Equipment Co., Ltd, catalog number: B030003)
Sealing film (Parafilm, catalog number: pm996)
Microscope slides, 76 × 26 mm (Sangon Biotech, catalog number: F518101)
Cover slides, 24 × 60 mm (Sangon Biotech, catalog number: F518118)
Rice seeds (Shuhui 527, indica)
Glycerol (Sangon Biotech Co., Ltd, catalog number: A501745-0500)
Mounting medium (Thermo Fisher, catalog number: 008030)
NH4Cl (Sigma, catalog number: A9434-500G)
Ca(NO3)2·4H2O (Sigma, catalog number: C1396-500G)
MgSO4·7H2O (Sigma, catalog number: M2773-500G)
K2SO4 (Sigma, catalog number: P0772-250G)
NaH2PO4·2H2O (Sigma, catalog number: 71500-250G)
FeSO4·7H2O (Sigma, catalog number: F7002-500G)
Na2EDTA·2H2O (Sigma, catalog number: 03685-500G)
H3BO3 (Sigma, catalog number: B0394-100G)
ZnSO4·7H2O (Sigma, catalog number: Z0251-500G)
CuSO4·5H2O (Sigma, catalog number: 209198-250G)
MnCl2·4H2O (Sigma, catalog number: M3634-100G)
(NH4)6·Mo7O24·4H2O (Sangon Biotech, catalog number: A600067-0100)
Formaldehyde (Sigma-Aldrich, catalog number: F8775)
Acetic acid (Sigma-Aldrich, catalog number: A6283)
Na2HPO4·12H2O (Sangon Biotech, catalog number: A607793)
Ethanol (any manufacturer)
Culture solution (see Recipes)
FAA stationary fluid (see Recipes)
0.2 M PBS (pH 7.0) (see Recipes)
Equipment
Constant-temperature incubator (Shanghai Jing Hong Laboratory Instrument Co. Ltd., catalog number: GNP-9080)
LED illumination (Fujian Jiupo Biotechnology Co. Ltd., catalog number: WL50)
Spectrum analyzer (EVERFINE, catalog number: PLA-30)
Eppendorf Research® plus Pipette (100–1,000 μL pipette, catalog number: 3120000062)
Vacuum (LABCONCO, CentriVap DNA vacuum concentrator, catalog number: 7970037)
Optical microscope (Nikon, model: ECLIPSE Ni-U)
Software
ImageJ (National Institutes of Health, https://imagej.nih.gov/ij/index.html)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Wu, H., Wang, Z., Yu, Y. and Zhang, Y. (2023). Imaging of Chloroplast Movement Responses to Light Stimulation in Different Intensities in Rice. Bio-protocol 13(4): e4611. DOI: 10.21769/BioProtoc.4611.
- Zhang, Y., Dong, G., Wu, L., Wang, X., Chen, F., Xiong, E., Xiong, G., Zhou, Y., Kong, Z., Fu, Y., et al. (2022). Formin protein DRT1 affects gross morphology and chloroplast relocation in rice. Plant Physiol. doi: 10.1093/plphys/kiac427.
分类
植物科学 > 植物细胞生物学 > 细胞成像
生物科学 > 生物技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link