- EN - English
- CN - 中文
Continuous Measurement of Reactive Oxygen Species Formation in Bacteria-infected Bone Marrow–derived Macrophages Using a Fluorescence Plate Reader
使用荧光酶标仪连续测量受细菌感染的骨髓源性巨噬细胞中活性氧的生成
(*contributed equally to this work) 发布: 2023年02月05日第13卷第3期 DOI: 10.21769/BioProtoc.4604 浏览次数: 2465
评审: Andrea PuharDhiman Sankar PalJunsik SungSaskia F. Erttmann

相关实验方案

在平板检测仪中使用高特异性荧光探针定量巨噬细胞细胞二价铁 (Fe2+) 含量
Philipp Grubwieser [...] Christa Pfeifhofer-Obermair
2024年02月05日 2226 阅读

研究免疫调控血管功能的新实验方法:小鼠主动脉与T淋巴细胞或巨噬细胞的共培养
Taylor C. Kress [...] Eric J. Belin de Chantemèle
2025年09月05日 3550 阅读
Abstract
Macrophages are at the center of innate immunity and are the main target cells of the intracellular pathogen Salmonella enterica serovar Typhi. The production of reactive oxygen and nitrogen species (ROS/RNS) is the host’s early response to invading microbes, as oxidative stress is highly toxic for bacteria. Adequate ROS/RNS production in infected macrophages is critical for the clearance of intracellular pathogens; this is achieved by several enzymes, including inducible NADPH phagocyte oxidase (NOX) and nitric oxide synthase (iNOS), respectively. The pro-inflammatory cytokine interferon gamma (IFNγ), primarily produced by activated natural killer cells and T-helper cells type 1, is a potent inducer of iNOS. Therefore, it is crucial for infection control through oxidative microbicidal activity.
To characterize the early oxidative stress response via ROS formation, which is critical for the reduction of Salmonella proliferation within macrophages, we established an in vitro model of murine macrophages infected with Salmonella enterica serovar Typhimurium (S.tm). This serovar induces a systemic infection in mice that is frequently used as a model for typhoid fever, which, in human subjects, is caused by Salmonella Typhi.
We generated bone marrow–derived macrophages (BMDM) from C57BL/6N wildtype mice using macrophage colony-stimulating factor (M-CSF) stimulation for six days. Thereafter, we infected BMDM with S.tm for one hour. Shortly before infection, cells were stained with CellROXTM Deep Red reagent. In its reduced form, CellROXTM is non-fluorescent. As a result of oxidation by ROS, this reagent exhibits strong fluorescence and persists within the cells. Subsequently, changes as a result of the oxidative stress response can be measured with a TECAN Spark microplate reader over time.
We designed this protocol to measure oxidative stress in macrophages through the course of an infection with an intracellular bacterium. The protocol has several advantages over established techniques. First, it allows to continuously monitor and quantify ROS production in living cells from the very start of the infection to the final clearance of the intracellular pathogen. Second, this protocol enables efficient ROS detection without stressing the cells by detaching or staining procedures.
Graphical abstract

Background
The Gram-negative enteric bacterium Salmonella enterica serovar Typhi causes typhoid fever in humans, which is a major cause of disability and death worldwide (Stanaway et al., 2019). The closely related Salmonella enterica serovar Typhimurium (Salmonella Typhimurium, S.tm) induces self-limiting gastroenteritis in humans and systemic infection in mice—a commonly used typhoid fever model.
As an intracellular pathogen, S.tm is capable of actively invading various cell types, but its virulence is dependent on its ability to selectively infect and proliferate inside macrophages (Fields et al., 1986; Haraga et al., 2008). Upon phagocytosis, S.tm establishes a replicative niche within a membrane-bound compartment termed the Salmonella-containing vacuole. There, the pathogen competes with various bactericidal mechanisms deployed by the host cell. One factor pivotal for initial inhibition of bacterial proliferation as well as pathogen clearance is the production of reactive oxygen and nitrogen species (ROS/RNS) (Vazquez-Torres et al., 2000; Herb and Schramm, 2021). In response to S.tm infection, macrophages activate several innate immune pathways that lead to ROS/RNS production and thus induction of bactericidal oxidative stress. A crucial component of the innate immune response identified is the membrane-bound enzyme NADPH oxidase (NOX), with the isoform NOX2 being at the center of antimicrobial ROS generation (Herb and Schramm, 2021). The importance of this enzyme is highlighted by the increased susceptibility of NOX2-deficient mice to infection with normally avirulent S.tm strains (Felmy et al., 2013). Another source of macrophage antimicrobial oxidative stress is mitochondria-augmented ROS production, which is directly linked to Toll-like receptor activation (West et al., 2011). Finally, the activity of inducible nitric oxide synthase (iNOS), crucial for pathogen control, is positively regulated by inflammatory cytokines, including interferon gamma (IFNγ) (Mastroeni et al., 2000). In multiple infection models, IFNγ has thus been implicated in adequate ROS/RNS production and enhancement of intracellular bacterial clearance in macrophages (Mastroeni et al., 2000; Nairz et al., 2008; Brigo et al., 2021). This antimicrobial effect of ROS/RNS is not only attributed to direct toxicity to the pathogen, but also to indirect effects, with oxidative stress being implicated in numerous cellular signaling pathways (Tan et al., 2016). Especially for the multi-faceted role of oxidative stress in innate immune defense, iNOS activation is deemed critical for the activation of nuclear factor erythroid 2–related factor-2 (Nrf2)-dependent pathways, which lead to a nutritional immune response aimed at limiting essential nutrients to the pathogen’s compartment (Nairz et al., 2013). The importance of ROS/RNS-mediated pathogen control is also evident in patients lacking functional ROS induction due to chronic granulomatous disease, as they are at high risk for invasive or recurrent forms of salmonellosis (Burniat et al., 1980;Mouy et al., 1989;Dinauer, 2005).
Due to its central involvement in innate immunity, an accurate measurement of oxidative stress is vital to study host–pathogen interactions. Herein, we report a method that allows to continuously determine ROS formation during infections in vitro. Most of the already established methods, which include usage of ROS-sensitive probes or biosensors expressed in cells or bacteria, rely on flow cytometry or fluorescence imaging techniques to quantify ROS levels (van der Heijden et al., 2015; Grander et al., 2022; Leone et al., 2016). These methods typically allow measurements only at single time points, as samples must be fixed and/or mounted. Furthermore, extensive preparation procedures will likely stress cells and thus decrease the signal-to-noise ratio. Of note, our novel protocol enables efficient detection of oxidative stress over time without stressing the cells by detaching and/or staining procedures. Furthermore, as fluorescence is read in a plate reader, multiple controls and experimental conditions can be quantified simultaneously. The protocol presented herein allows the immediate and continuous monitoring and quantification of oxidative stress responses during infection of macrophages with intracellular microbes (Figure 1).
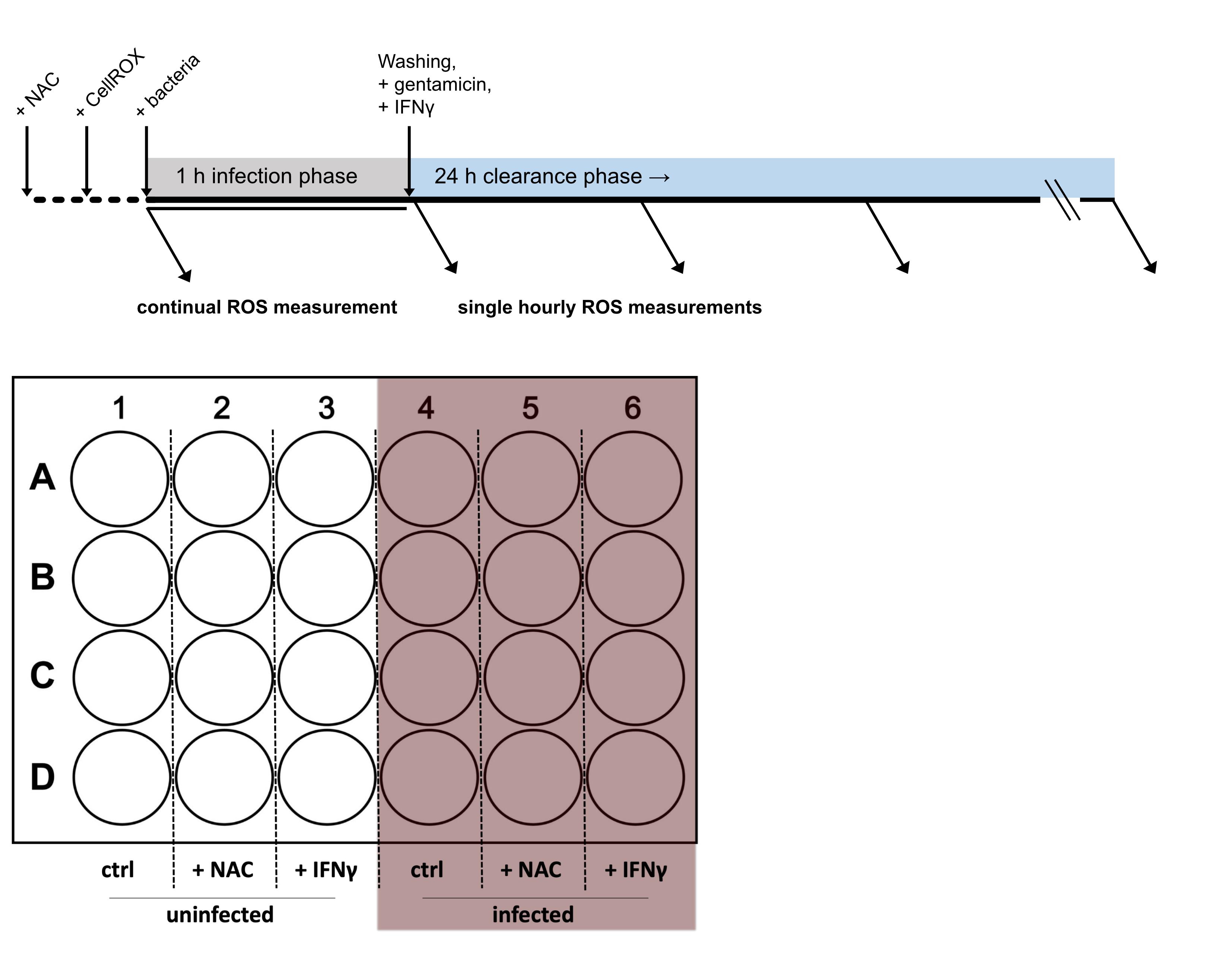
Figure 1. Timeline and 24-well plate layout of the experimental procedure
Materials and Reagents
250 mL Erlenmeyer flask (Stoelzle Medical, catalog number: 21226368000)
0.5 mL Eppendorf tubes (Eppendorf, catalog number: 0030121.023)
50 mL polypropylene tube (Falcon, catalog number: 352070)
24-well plate (Falcon, catalog number: 353047)
Cell scraper (Sarstedt, catalog number: 83.3951)
LunaTM cell counting slides (Biocat, catalog number: L201B1C3GB)
Disposable cuvette (BRAND, catalog number: 759015)
15 cm cell culture dish (Falcon, catalog number: 353025)
5 mL disposable syringe (BD, catalog number: 309050)
10, 200, and 1,250 µL pipette tips (STARLAB, catalog number: S1110-3700, S1120-3810, and S1112-1720, respectively)
5, 10, and 25 mL disposable pipettes (Falcon, catalog number: 606180, 607180, and 357525, respectively)
Disposable hypodermic needle 100 Sterican R (Braun, catalog number: 4657519)
Cell strainer 40 µm (Falcon, catalog number: 352360)
Salmonella enterica serovar Typhimurium ATCC14028 (ATCC)
Lysogeny broth (LB) medium Lennox (Roth, catalog number: X964.2)
Glycerol (Sigma, catalog number: G5516-100ML)
Phosphate buffer saline (PBS) (Lonza, catalog number: 17-515 F)
Disposable cuvette (BRAND, catalog number: 759015)
Casy cup (OMNI Life Science, catalog number: 5651794)
Casy Ton buffer (OMNI Life Science, catalog number: 5651808)
Aqua bidest (Fresenius Kabi, catalog number: 16.231)
Pen-Strep (Lonza, catalog number: DE17-602E)
Gentamicin (Gibco, catalog number: 15750-037, stock: 50 mg/mL)
L-glutamine (Lonza, catalog number: BE17-605E)
Dulbecco′s modified Eagle′s medium (DMEM) (Pan BiotechTM, catalog number: P04-01500)
Fetal bovine serum (FBS) (Pan BiotechTM, catalog number: P30-3031)
CellROXTM Deep Red (Thermo Fisher, catalog number: C10422)
N-acetylcysteine (NAC) (Sigma-Aldrich, catalog number: A7250)
Acridine orange/propidium iodide stain (Biocat, catalog number: F23001)
Recombinant murine interferon-gamma (Peprotech, catalog number: 315-05; stock 20 µg/mL)
Recombinant murine colony-stimulating factor M-CSF (Peprotech, catalog number: 315-02)
Ketamine (Livisto, catalog number: 6680219)
Xylazine (Animedica, catalog number: 7630517)
Omnican F syringes (Braun, catalog number: 91615025)
M-Lyse buffer concentrate (10×) (erythrocyte lysis kit) (R&D, catalog number: WL2000)
LB medium (see Recipes)
LB medium with 30% glycerol (see Recipes)
Cell culture medium (see Recipes)
Cell culture medium for infection with S.tm (see Recipes)
Mouse line
Bone marrow cells were collected from female C57BL/6N mice, which were bred in the Animal facility of the Medical University of Innsbruck or ordered from Charles River Laboratories.
Equipment
Pipetman L Starter kit: 2, 20, 200, and 1,000 µL pipettes (GILSON, catalog number: F167370)
Sartorius Midi Plus pipetting controller (Sartorius, catalog number: 710931)
Biosafety level 2 laminar flow cabinet, EuroClone Sicherheitswerkbank Safe Mate Eco 1.2 (Politakis Laborgeräte, catalog number: EN 12 469)
Shaking incubator (VWR, catalog number GFL 3031)
Heraeus® HERAcell® CO2 Incubator (Thermo Fisher Scientific)
Photometer (Eppendorf, catalog number: BioPhotometer D30)
Centrifuge (Hettich Micro 200R and Rotanta 460R)
Casy counting system (OMNI Life Science)
Automated multimode microplate reader (TECAN Spark)
LUNA automated cell counter (Biocat, catalog number: L10001-LG)
Millivac-Maxi vacuum pump (Merck, catalog number: SD1P014M04)
Software
SparkControlTM (TECAN Trading, Ltd.)
GraphPad Prism 9.1 (GraphPad Software)
Procedure
文章信息
版权信息
© 2023 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Brigo, N., Grubwieser, P., Theurl, I., Nairz, M., Weiss, G. and Pfeifhofer-Obermair, C. (2023). Continuous Measurement of Reactive Oxygen Species Formation in Bacteria-infected Bone Marrow–derived Macrophages Using a Fluorescence Plate Reader. Bio-protocol 13(3): e4604. DOI: 10.21769/BioProtoc.4604.
分类
免疫学 > 免疫细胞功能 > 巨噬细胞
生物化学 > 其它化合物 > 活性氧
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










