- EN - English
- CN - 中文
Infection of the Developing Central Nervous System of Drosophila by Mammalian Eukaryotic and Prokaryotic Pathogens
哺乳动物真核和原核病原体对果蝇发育中枢神经系统的感染
发布: 2022年12月05日第12卷第23期 DOI: 10.21769/BioProtoc.4563 浏览次数: 2032
评审: Nafisa M. JadavjiKai YuanAnonymous reviewer(s)
Abstract
Pathogen invasion of the central nervous system (CNS) is an important cause of infection-related mortality worldwide and can lead to severe neurological sequelae. To gain access to the CNS cells, pathogens have to overcome the blood–brain barrier (BBB), a protective fence from blood-borne factors. To study host–pathogen interactions, a number of cell culture and animal models were developed. However, in vitro models do not recapitulate the 3D architecture of the BBB and CNS tissue, and in vivo mammalian models present cellular and technical complexities as well as ethical issues, rendering systematic and genetic approaches difficult. Here, we present a two-pronged methodology allowing and validating the use of Drosophila larvae as a model system to decipher the mechanisms of infection in a developing CNS. First, an ex vivo protocol based on whole CNS explants serves as a fast and versatile screening platform, permitting the investigation of molecular and cellular mechanisms contributing to the crossing of the BBB and consequences of infection on the CNS. Then, an in vivo CNS infection protocol through direct pathogen microinjection into the fly circulatory system evaluates the impact of systemic parameters, including the contribution of circulating immune cells to CNS infection, and assesses infection pathogenicity at the whole host level. These combined complementary approaches identify mechanisms of BBB crossing and responses of a diversity of CNS cells contributing to infection, as well as novel virulence factors of the pathogen.
Graphical abstract

Procedures flowchart. Mammalian neurotropic pathogens could be tested in two Drosophila central nervous system (CNS) infection setups (ex vivo and in vivo) for their ability to: (1) invade the CNS (pathogen quantifications), (2) disturb blood–brain barrier permeability, (3) affect CNS host cell behaviour (gene expression), and (4) alter host viability.
Background
Infections of the central nervous system (CNS) are devastating yet remain relatively rare due to the presence of specific protective layers. In particular, the blood–brain barrier (BBB) is a selective physical and chemical filter, and performs its neuroprotective role by controlling molecular import into the CNS and blocking the entry of circulating cells (Daneman and Prat, 2015). In higher vertebrates, brain microvascular endothelial cells, harbouring intercellular tight junctions, form the core structure of the BBB. In addition, pericytes, astrocytes, and an extracellular matrix basal membrane regulate BBB integrity and functions (Obermeier et al., 2013; Daneman and Prat, 2015; Saunders et al., 2016). However, several pathogens have developed strategies to overcome the BBB and invade the CNS (Coureuil et al., 2017). To study interactions between pathogens and the BBB, various cell culture systems have been developed, from simple endothelial monolayers to more complex multicellular cultures, combining endothelial cells and pericytes (Sivandzade and Cucullo, 2018). While these in vitro models have proposed a number of BBB crossing mechanisms, they remain limited in recapitulating the 3D architecture of the BBB and its interactions with the complexity of CNS cells (Sivandzade and Cucullo, 2018; Jackson et al., 2019). Moreover, organoids also present architectural limitations (Bergmann et al., 2018). To bypass this restriction, a number of animal models, in particular rodents and zebrafish, have been used (Dando et al., 2014; Jackson et al., 2019), allowing the investigation of infection mechanisms in vivo. However, in addition to cost and ethical issues, their genetic and cellular complexity prevents systematic, screen-based approaches. Separating the systemic from the local impact of these immune challenges is also difficult in such context, and infections can have lethal consequences for the host. In addition, culturing CNS tissue of these animal models is still difficult.
Drosophila melanogaster represents an original and powerful model to study CNS infection, from identifying novel mechanisms of BBB-pathogen interactions to understanding the extent of consequences on the diversity of CNS cell populations. The fly BBB is composed of two glial layers exhibiting conserved neuroprotective mechanisms with the mammalian BBB. The subperineurial glia (SPG), forming an epithelium-like structure with septate junctions, represent the core structure and physical filter of the BBB (Stork et al., 2008; Hindle and Bainton, 2014; Weiler et al., 2017; Babatz et al., 2018). The perineurial glia, a hemolymph sensor, and the extracellular matrix both cover the SPG (DeSalvo et al., 2014; Parkhurst et al., 2018). Many transporter proteins expressed by the BBB to allow the selective shuttling of diverse solutes and xenobiotics are actually conserved, including solute carriers, ATP-binding cassette transporters, and lipoprotein receptors (DeSalvo et al., 2014; Hindle and Bainton, 2014; Weiler et al., 2017). Moreover, many aspects of mammalian neurogenesis are conserved in flies (Mira and Morante, 2020), enabling us to probe the impact of infection on such process. Drosophila neural stem cells self-renew via asymmetric cell division, switch from quiescence to proliferation, and are present within a specific microenvironment sharing neurogenic features and common constituents with the mammalian niche, including glial populations and the BBB (Homem and Knoblich, 2012; Otsuki and Brand, 2017). Finally, due to Drosophila unrivalled genetics, each of the BBB cell layers and the different CNS populations can be manipulated independently, in parallel with each other, and in a temporally controlled and spatially restricted manner. These features provide a simpler, genetically tractable model to study CNS infection, from pathogen entry to downstream consequences for the host.
We have devised a double, complementary approach to generate CNS infection in Drosophila:
An ex vivo protocol that mimics CNS infection. Here, an intact larval CNS explant is cultured in a specific medium inoculated with pathogens (Benmimoun et al., 2020). This explant setup is first designed to allow fast screening of pathogens, mutant strains, and culture conditions for the generation and outcome of CNS infection. In this frame, it has first allowed the identification of a number of prokaryotic and eukaryotic neurotropic pathogens, known to trigger meningitis and/or encephalitis in mammals, that are able to cross the fly BBB. On the prokaryotic side, this included Streptococcus agalactiae (Group B Streptococcus, GBS), Streptococcus pneumoniae, Listeria monocytogenes, and the strictly human Neisseria meningitidis. The eukaryotic Candida albicans and Candida glabrata were also found to reach the Drosophila CNS, but not Cryptococcus neoformans. This ex vivo platform was further used to screen a collection of GBS mutant strains, by which surface lipoproteins were identified—more precisely the lipoprotein Blr—as virulence factors crucial for BBB crossing (Benmimoun et al., 2020). Finally, its combination with Drosophila genetics revealed the Drosophila lipoprotein receptor (LpR2), which is expressed on the surface of the BBB layer on the SPGs, as a host receptor for Blr, and important for CNS invasion by GBS (Benmimoun et al., 2020).
Another asset of the explant protocol is to study the effect of pathogenic infection on the different cell populations outside of the systemic context (for example, outside of systemic inflammation, a common hallmark of infection), potentially revealing other layers of response that could be otherwise hidden. For example, the lactic acidosis generated in the milieu by GBS could be quenched by buffer solutions, revealing the contribution of this phenomenon to BBB weakening (Benmimoun et al., 2020).
An in vivo setup through direct microinjection of the pathogen into the fly circulatory system (Benmimoun et al., 2020; Winkler et al., 2021). This approach aims to evaluate the impact of infection i) on the CNS, this time including the contribution of systemic components, such as circulating immune cells, and ii) at the level of the whole organism, by assessing a diversity of host–pathogen interactions, in particular virulence. Comparison with the explant system allows to confirm or infirm the importance of molecular and cellular mechanisms in a whole-body context, offering the possibility to discriminate between local and systemic effects, and to identify the hierarchy between and dominance of specific mechanisms.
This protocol was first used to determine the conservation of GBS Blr and Drosophila LpR2 interactions in BBB crossing mechanism and CNS invasion, initially identified through the explant setup (Benmimoun et al., 2020). Moreover, assessing survival overtime of the injected larvae in this setup demonstrated that Blr is a virulence factor in Drosophila, a property further validated in a mouse model of GBS hematogenous brain infection (Benmimoun et al., 2020). This whole in vivo CNS infection protocol also participated in revealing a new inflammatory mechanism, in which glial cells direct CNS infiltration by macrophages in response to GBS infection (Winkler et al., 2021).
Here, we detail these two complementary platforms to elicit infection of the Drosophila developing CNS by mammalian neurotropic pathogens. By taking advantage of the Swiss knife of Drosophila genetics, the architectural preservation of the CNS tissue, and the similarity of many fundamental cellular and molecular mechanisms with mammals, these approaches allow more systematic screening of wild-type and mutant pathogen strains, as well as targeted genetic manipulations, to infer precise causal relationships between cellular layers or between molecular pathways during infection. Ultimately, these setups can be used to study all steps of the CNS infection, from crossing of the BBB to downstream consequences on the host, including neuroinflammation and impact on neurogenesis.
Although these methods are described for prokaryotic and eukaryotic pathogens, they could be adapted for other pathogens (virus, parasites) as well as for studying systemic extrinsic stresses, and for investigating the entry of therapeutic molecules into the CNS.
Materials and Reagents
Fly strain and Drosophila larval culture
mdr65-mtd: Tomato (Benmimoun et al., 2020 and Figure 1)
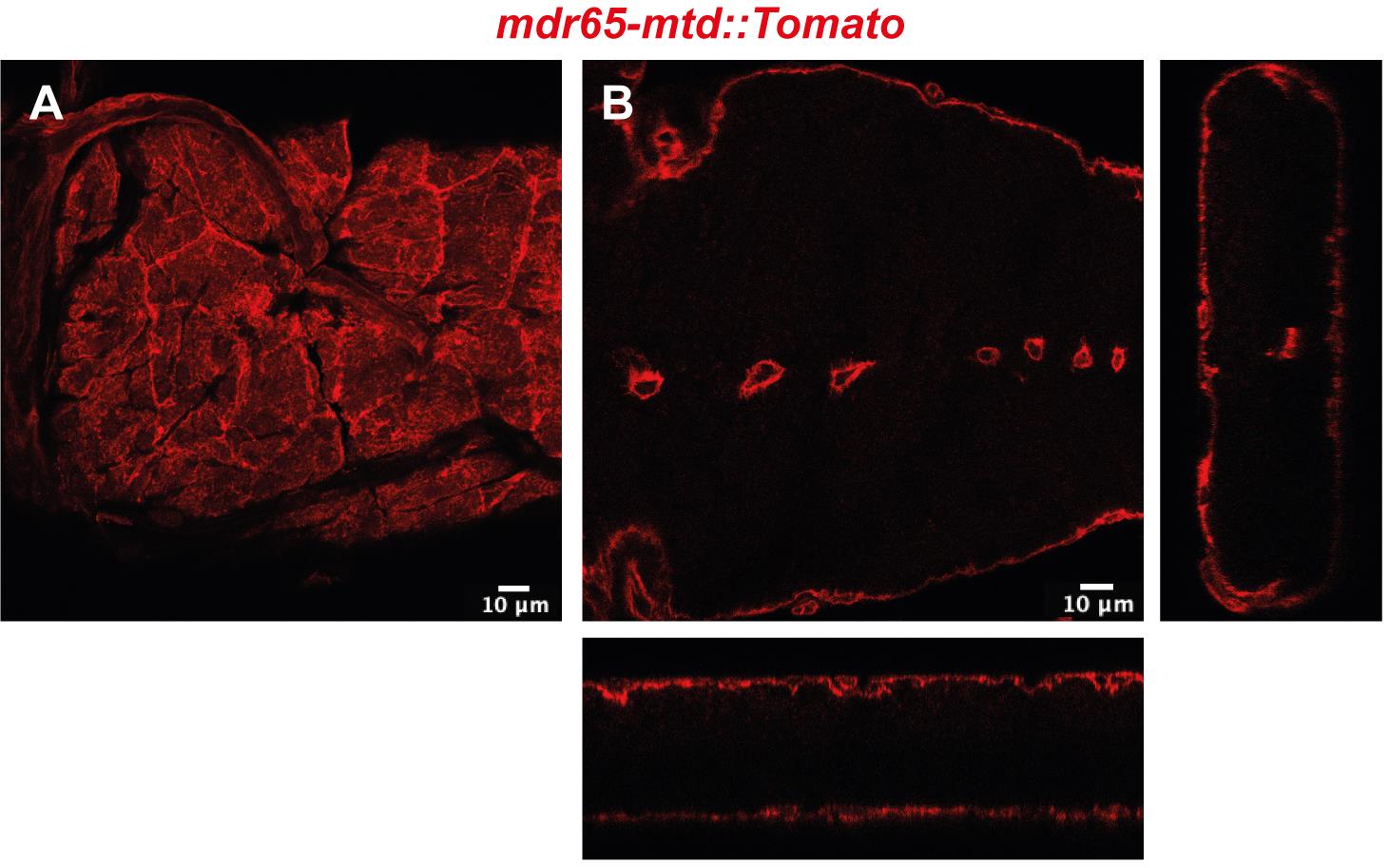
Figure 1. Confocal image of top view (A) and cross-section with orthogonal views (B) of the Drosophila BBB in a portion of the ventral nerve cordEgg laying plates (50 mm, Deep) (Thermo Scientific, catalog number: 124-17), see Figure 2A
Fly egg laying cages (homemade, see Figure 2B)
Larval collection plates (60 mm Petri dishes) (Corning-Falcon, catalog number: 353004), see Figure 2C
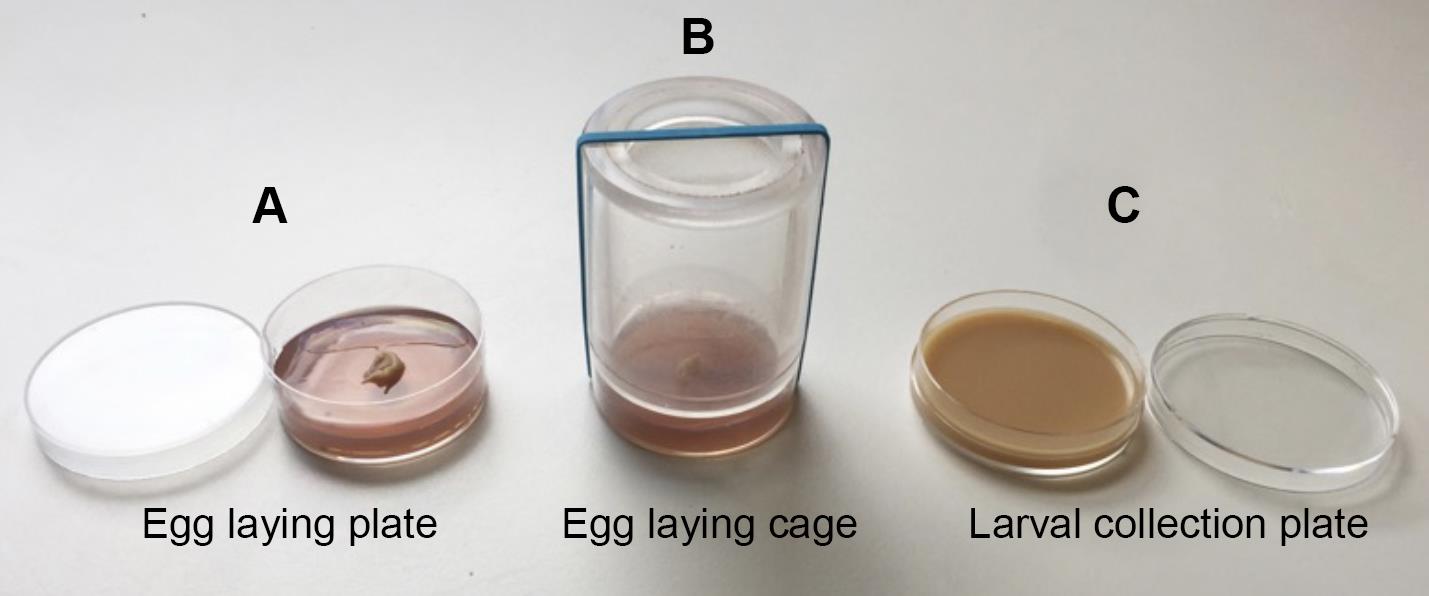
Figure 2. Fly larval collection and culture materialsActive dry yeast (Genesee Scientific, catalog number: 62-103)
Corn flour (Limagrain, catalog number: WFMZ01HS)
Agar (Sigma-Aldrich, catalog number: A7002-1KG)
Sugar (Dominique Dutscher, catalog number: 067508B)
Organic grape juice
Propionic acid (Sigma-Aldrich, catalog number: P5561-1L)
Methyl-4-hydroxybenzoate (Sigma-Aldrich, catalog number: H3647)
Ethanol (Sigma-Aldrich, catalog number: 24105)
Egg laying medium (see Recipes)
Fly food (see Recipes)
Pathogen preparation
Pathogen growth media [Brain Heart Infusion broth (BHI), Yeast extract Peptone Dextrose (YPD), or de Man Rogosa Sharpe (MRS)] (BD Difco, Millipore, catalog numbers: 237500, 242820 or 288130)
Round-bottom tubes with cap (Fisher Scientific, Falcon, catalog number: 352059)
Micro cuvette for spectrophotometer (Dutscher, Greiner Bio-One, catalog number: 613101)
Microcentrifuge tubes (Fisher Scientific, Eppendorf, catalog number: 10708704)
Dulbecco’s phosphate-buffered saline (PBS) (Sigma-Aldrich, catalog number: D1408)
Drosophila Schneider’s medium (Fisher Scientific, Gibco, catalog number: 21720-024)
Drosophila dissection
Fine forceps (Fine Science Tools, Dumont #5, catalog number: 11252-20)
Tissue culture dishes (Fisher Scientific, Corning, Falcon, catalog number: 3530001)
Glass cavity dish (Atom Scientific, catalog number: SDCE4040-1)
Dulbecco’s phosphate-buffered saline (PBS) (Sigma-Aldrich, catalog number: D1408)
Ethanol (Sigma-Aldrich, catalog number: 493511)
Drosophila Schneider’s medium (Fisher Scientific, Gibco, catalog number: 21720-024)
CNS explant culture
24-well cell culture plate (Fisher Scientific, Corning Falcon, catalog number: 353047)
Drosophila Schneider’s medium (Fisher Scientific, Gibco, catalog number: 21720-024)
l-Glutamine (Fisher Scientific, Gibco, catalog number: 25030-032)
Sodium l-ascorbate (Sigma-Aldrich, catalog number: A4034)
Fetal bovine serum (Sigma-Aldrich, catalog number: F4135)
CNS culture medium I (see Recipes)
CNS culture medium II (see Recipes)
In vivo CNS infection
Fine forceps (Fine Science Tools, Dumont #5, catalog number: 11252-20)
Pipette Drummond Scientific 3.5’’ (Dutsher, Drummond Scientific, catalog number: 3-000-203-G/X)
Nano-injector Nanoject III (Dutsher, Drummond Scientific, catalog number: 075250)
Mineral oil (Sigma-Aldrich, catalog number: M5904)
Microscope slides (Fisher Scientific, Fisherbrand, catalog number: 10219280)
Permeability assay
Drosophila Schneider’s medium (Fisher Scientific, Gibco, catalog number: 21720-024)
Dextran, Texas Red, lysine fixable 10 KDa (ThermoFisher, catalog number: 11530236, Invitrogen D1863)
Dulbecco’s phosphate-buffered saline (PBS) (Sigma-Aldrich, catalog number: D1408)
4% methanol-free formaldehyde (ThermoFisher Scientific, catalog number: 28908)
Immunohistochemistry
4% methanol-free formaldehyde (ThermoFisher Scientific, catalog number: 28908)
Bouin’s solution (Sigma-Aldrich, catalog number: HT10132)
Dulbecco’s phosphate-buffered saline (PBS) (Sigma-Aldrich, catalog number: D1408)
Triton X-100 (Sigma-Aldrich, catalog number: T9284)
Bovine serum albumin (Sigma-Aldrich, catalog number: A3608)
Normal goat serum (Invitrogen, catalog number: 10000C)
Mowiol mounting medium (homemade), or equivalent
Microscope slides (Fisher Scientific, Fisherbrand, catalog number: 10219280)
Coverslips (Fisher Scientific, Fisherbrand, catalog number: 15707592)
Anti-GBS antibody (Benmimoun et al., 2020)
Gene expression
Fine forceps (Fine Science Tools, Dumont #5, catalog number: 11252-20)
Dulbecco’s phosphate-buffered saline (PBS) (Sigma-Aldrich, catalog number: D1408)
Glass cavity dish
RNeasy Mini kit (QIAGEN, catalog number: 74104)
QuantiTect Rev. Transcription Kit (QIAGEN, catalog number: 205311)
TaqMan gene expression assay (ThermoFisher, catalog number: 4331182)
TaqMan Universal PCR Master Mix II (ThermoFisher, catalog number: 4440042)
96-well PCR plates (e.g., Bio-Rad, catalog number: MLL9601)
Seals for PCR plate (e.g., Bio-Rad, catalog number: MSB1001)
Equipment
Incubator with regulated temperature and humidity (Memmert, HPP110, or equivalent)
Agitator Titramax 100 (Heidolph Instruments, catalog number: 544-11200-00)
Spectrophotometer (Pharmacia, Novaspec II)
Centrifuge (Sigma Bioblock scientific, 1-15K)
Microbiological Safety Post (HeraSafe KS9)
Flaming/Brown micropipette puller (Sutter Instrument Model P-97)
Nano-injector Nanoject III (Dutsher, Drummond Scientific, catalog number: 075250)
Laser scanning confocal microscope [Zeiss LSM 880 with Zen software (2012 S4)]
Real-Time PCR machine (e.g., ThermoFisher, StepOne Real-Time PCR System, catalog number: 4376357)
Software
Zen software (2012 S4)
Fiji (ImageJ version 2020 2.1.0/1,53c and version 1.52p; available at: https://imagej.net/software/fiji/)
GraphPad Prism software [version 7 and version 2020 8.4.2 (464)]
R, SPSS, or comparable software
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Benmimoun, B., Winkler, B. and Spéder, P. (2022). Infection of the Developing Central Nervous System of Drosophila by Mammalian Eukaryotic and Prokaryotic Pathogens. Bio-protocol 12(23): e4563. DOI: 10.21769/BioProtoc.4563.
分类
神经科学 > 发育
微生物学 > 微生物-宿主相互作用 > 离体模型
生物科学 > 生物技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link