- EN - English
- CN - 中文
Hypochlorite Stress Assay for Phenotypic Analysis of the Halophilic Archaeon Haloferax volcanii Using an Improved Incubation Method and Growth Monitoring
利用改进的培养方法和生长监测对嗜盐火山生菌进行表型分析的次氯酸盐胁迫试验
发布: 2022年11月20日第12卷第22期 DOI: 10.21769/BioProtoc.4557 浏览次数: 1560
评审: Gal HaimovichYufang LuWolf Dieter Röther
Abstract
The study of haloarchaea provides an opportunity to expand understanding of the mechanisms used by extremophiles to thrive in and respond to harsh environments, including hypersaline and oxidative stress conditions. A common strategy used to investigate molecular mechanisms of stress response involves the deletion and/or site-directed mutagenesis of genes identified through omics studies followed by a comparison of the mutant and wild-type strains for phenotypic differences. The experimental methods used to monitor these differences must be controlled and reproducible. Current methods to examine recovery of halophilic archaea from extreme stress are complicated by extended incubation times, nutrients not typically encountered in the environment, and other related limitations. Here we describe a method for assessing the function of genes during hypochlorite stress in the halophilic archaeon Haloferax volcanii that overcomes these types of limitations. The method was found reproducible and informative in identifying genes needed for H. volcanii to recover from hypochlorite stress.
Keywords: Archaea (古细菌)Background
Accumulation of reactive species that are redox-active compounds usually leads to cytotoxic activity. Hypochlorite (HOCl) is a reactive species that is particularly cytotoxic as it reacts in vivo with low molecular weight inorganic molecules and organic molecules including the functional groups of lipids, proteins, carbohydrates, and nucleic acids (Panasenko et al., 2013). HOCl levels usually become more abundant during oxidative stress when there is an increase in molecular oxygen (O2) leading to incomplete reactions stopping at reactive oxygen species such as superoxide anions (O2•−), hydrogen peroxide (H2O2), and the highly reactive hydroxyl radical (OH•) (Loi et al., 2015). The rise in H2O2 levels leads to chlorination where H2O2 reacts with Cl– anions leading to the formation of HOCl (Winterbourn and Kettle, 2013).
H2O2 + Cl– + H+ → HOCl + H2O
HOCl ↔ OCl– + H+
While haloarchaea are of interest to understand how organisms thrive in harsh environments, mechanisms of HOCl stress response are best understood in pathogenesis, as neutrophils of mammalian innate immunity kill exogenous pathogens through an oxidative burst that includes HOCl production (Imlay, 2003, 2008, 2013; Ulfig and Leichert, 2021). Haloarchaea are microorganisms that often dominate hypersaline ecosystems where the concentration of NaCl is greater than in seawater (3.5% w/v NaCl) (Jones and Baxter, 2017). Of note in HOCl stress is the exceedingly high concentrations of chloride ions that are constantly present as exemplified by the hypersaline Lake Tyrrell that fluctuates from 4 to 5 M Cl– (Podell et al., 2014). These conditions lead to an increase in HOCl and consistent exposure of cells to cytotoxic agents. The study of haloarchaea is of interest to the scientific community, as these microorganisms can thrive in such harsh conditions. This inquiry has led to examining the mechanisms used by the haloarchaea to survive stress, including co-expression networks of coordinately regulated genes used to combat or control oxidative stress (Martinez-Pastor et al., 2017).
A common method used in biology to determine if a protein plays an important role in the cell is to delete the gene that encodes the protein of interest and observe the mutant strain phenotype. For example, here we are interested in comparing the growth rate and recovery of mutant and wild-type strains of the halophilic archaeon Haloferax volcanii before and after exposure to HOCl stress. For HOCl, supplementation of cultures with sodium hypochlorite (NaOCl) in aqueous solution leads to the spontaneous conversion of NaOCl into sodium hydroxide (NaOH) and HOCl, which can further dissociate into hydroxide (OH–) and hypochlorite (OCl–).
NaOCl + H2O ↔ NaOH + HOCl ↔ Na+ + OH– + H+ + OCl–
However, cultivating haloarchaea in a controlled environment can be difficult due to their demand for high concentrations of salts (e.g., > 2.5 M NaCl) and thermophilic temperatures (42–55 °C) (Robinson et al., 2005). Both environmental factors lead to evaporation, which exacerbates stress and can extend the time of cultivating cells for HOCl stress assays.
Here we describe a newly designed and improved method to examine the response of the haloarchaeon Haloferax volcanii to HOCl stress. Our initial analysis was conducted in a circular rotary shaker (Figure 1A) that yielded variable results. The inconstancy in results was likely due to the extensive incubation times required to detect cell recovery after HOCl exposure in minimal medium, as the conditions were microaerobic and dehydrating. These findings led to a demand for better growth conditions where the recovery of H. volcanii from HOCl stress could be monitored in a reproducible manner. Thus, a new protocol was developed that reduces the lag time of cellular recovery from HOCl stress and allows for experimental reproducibility by cultivating the cells using a mini rotator to improve aeration and redesigning the incubator to promote humidity (Figure 1B). The method is performed in minimal medium to avoid the complexity of antioxidants otherwise present in yeast extract and other common additions to undefined medium.
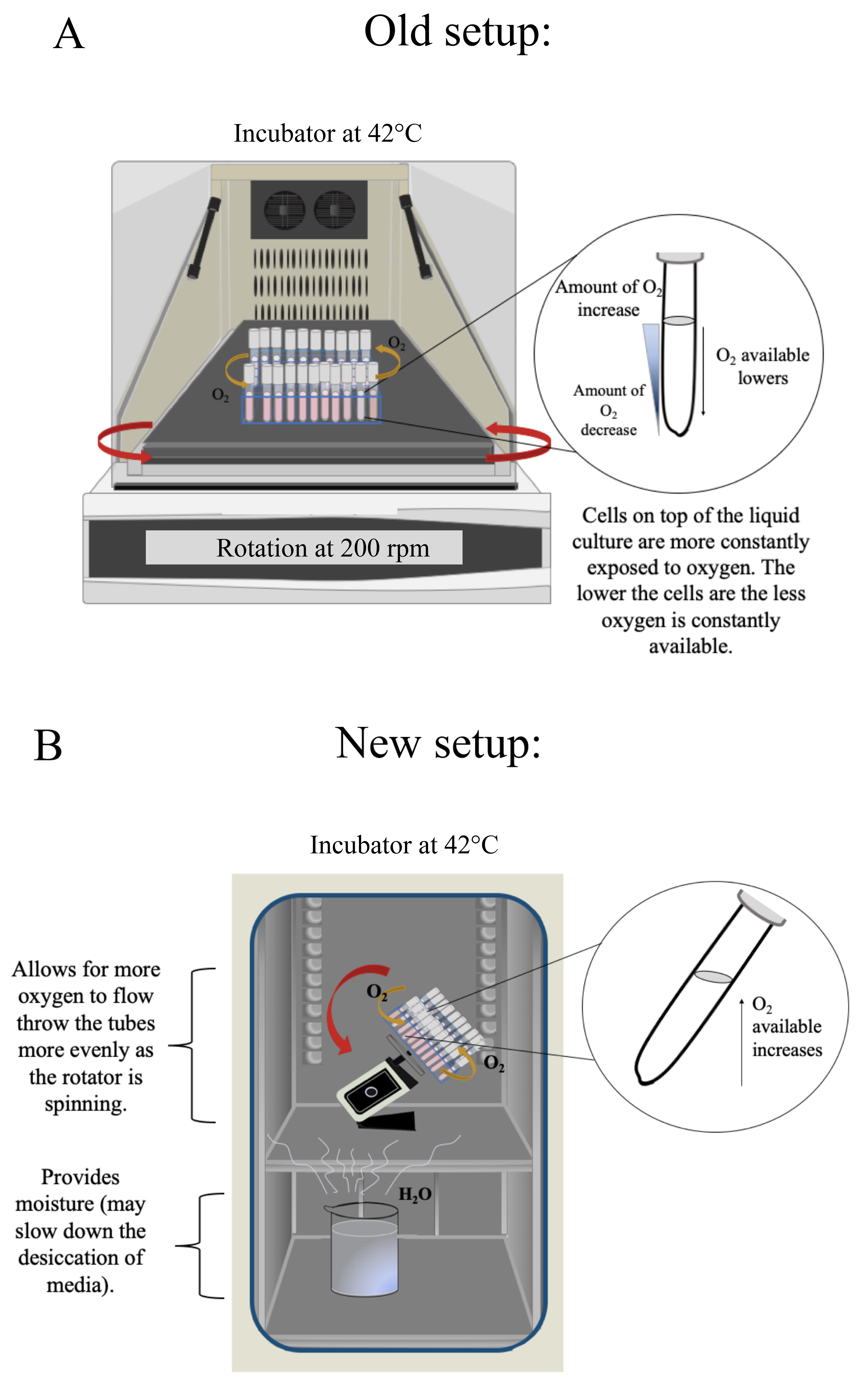
Figure 1. Diagram comparing two different setups for cell growth in culture tubes. A) Old setup with culture tubes oriented upright and agitated by rotary shaking, which causes an uneven distribution of oxygen and irregular growth patterns. B) New setup with culture tubes rotated at an angle to allow for more even aeration. A beaker is filled with water and included at the bottom of the incubator to enhance moisture.
Materials and Reagents
"Zipper" seal sample bags; thickness, 2 mil, and size, 6 × 9 in.
10 mL Sterile polystyrene disposable serological pipets (Genesee Scientific, catalog number: 12-104)
15 mL Conical centrifuge tubes, racked (polypropylene, Olympus Plastics, catalog number: 28-101)
2.5” Toothpicks, needle point, autoclavable (sterile) (LevGo, catalog number: 18250-NP)
200 µL XTIP4 barrier tips, low binding, racked, pre-sterilized (RNase and DNase free) (Genesee Scientific, catalog number: 24-712)
250 mL Erlenmeyer flasks (Pyrex, manufacturer number: 4980250/EMD) (Fisher Scientific, catalog number: S63271)
500 mL Erlenmeyer flasks (Pyrex, manufacturer number: 4980500/EMD) (Fisher Scientific, catalog number: S63273)
Disposable culture tubes, borosilicate glass 13 × 100 mm (Fisher Scientific, catalog number: 14-961-27)
Disposable plastic cuvettes semi-micro, 1.5 mL (Fisher Scientific, catalog number: 14955127)
Disposable petri dishes, polystyrene, sterile, semi-stackable (100 × 15 mm) (VWR, catalog number: 25384-088)
Plain PTFE stir bars, length: 70 mm; diameter: 10 mm (Fisher Scientific, catalog number: 16255801)
Plastic caps (to cap culture tubes) (DWK Life Sciences, manufacturer number: 7366013) (Fisher Scientific, catalog number: 14-957-91)
Poxygrid 96-place test tube rack; for 13–16 mm tubes (Bel-Art, catalog number: F18765-0001)
PYREX griffin low form 1 L beaker, double scale, graduated (Pyrex, manufacturer number: 10001L/EMD) (Fisher Scientific, catalog number: S14276)
Agar ash 2.0%–4.5% (Sigma-Aldrich, catalog number: A7002-250G)
Aluminum foil roll (used to cover opening for the Erlenmeyer flasks for sterilization and growing strains) (Fisher Scientific, catalog number: 01-213-105)
Ammonium chloride (NH4Cl) (Fisher Scientific, catalog number: A661-500)
Calcium chloride dihydrate (CaCl2·2H2O) (Fisher Scientific, catalog number: C79-500)
Copper sulfate pentahydrate (CuSO4·5H2O) (Sigma-Aldrich, catalog number: C3036)
D-Biotin (Fisher Bioreagents, catalog number: 58-85-5)
Glycerol for molecular biology (Fisher Bioreagents, catalog number: BP229-4)
Haloferax volcanii strains used including the H26 parent and SH125 mutant (ΔoxsR) that are previously described (Mondragon et al., 2022)
Iron sulfate heptahydrate (FeSO4·7H2O) (Alfa Aesar, catalog number: 7782-63-0)
L–Shaped cell spreader (Fisher Scientific, catalog number: 14-665-230)
Magnesium chloride hexahydrate (MgCl2·6H2O) (Sigma-Aldrich, catalog number: M0250-KG)
Magnesium sulfate heptahydrate (MgSO4·7H2O) (Fisher Science Education, catalog number: S25414A)
Manganese chloride tetrahydrate (MnCl2·4H2O) (Fisher Chemical, catalog number: M87-100)
Nanopure water (purified from Barnstead/Sybron Nanopure II 4-Module Water Purification System)
Novobiocin sodium salt (≥93% HPLC) (Sigma-Aldrich, catalog number: 74675-1G)
Potassium chloride (KCl) (Fisher Chemical, catalog number: P217-3)
Potassium phosphate dibasic anhydrous (K2HPO4) (Fisher Chemical, catalog number: P288-500)
Potassium phosphate monobasic (KH2PO4) (Fisher Chemical, catalog number: P285-500)
Potassium sulfate (K2SO4) (Fisher Chemical, catalog number: P304-500)
Sodium chloride (NaCl) certified ACS crystalline (Fisher Scientific, catalog number: S271-10)
Sodium hypochlorite (NaOCl) reagent grade, available chlorine 10%–15% (Sigma-Aldrich, catalog number: 425044-250mL)
Thiamine (Sigma cell culture, catalog number: T3902)
Tris-base (Fisher Bioreagents, catalog number: BP152-500)
Tryptone (Fisher Bioreagents, catalog number: BP1421-500)
Uracil, 99+% (Acros organics, catalog number: 66-22-8)
Yeast extract molecular genetics powder (Fisher Bioreagents, catalog number: BP1422-500)
Zinc sulfate heptahydrate (ZnSO4·7H2O) (Fisher Scientific, catalog number: 7446-20-0)
Concentrated salt water (SW) stock solution at 30% (w/v) (see Recipes)
GMM base (see Recipes)
Supplements (see Recipes)
0.5 M Potassium phosphate buffer (KPB), pH 7.5 for 100 mL total
1.5 mg/mL Uracil
Thiamine and biotin solution for 10.8 mL total
Hv-minimal salts for 12 mL total
Trace elements for 100 mL total
Volume of supplements to add to GMM base for total of 1 L of GMM + uracil (see Recipes)
For GMM + uracil agar plates a total of 500 mL (see Recipes)
For ATCC 974 medium plates a total of 500 mL (see Recipes)
Equipment
Cimarec basic stirring hotplate (ThermoFisher Scientific, catalog number: SP194715)
Genesys 40 visible spectrophotometer (ThermoFisher Scientific, catalog number: SP194715)
Genie 2 vortex mixer (Fisher Scientific, catalog number: 12-812)
Heratherm incubator (Thermo Scientific)
I24 Incubator shaker series (New Brunswick Scientific)
Mini rotator (20° angle, 2–80 rpm with disk and clamps, 120 V, Glas-Col Terre Haute, USA)
Pipette (10–100 µL, Rainin, pipet-lite)
Spectronic 20+ spectrophotometer (ThermoSpectronic, Filter: 600–950 nm)
Software
Microsoft Excel (version 16.16.27)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Mondragon, P., Hwang, S., Schmid, A. and Maupin-Furlow, J. A. (2022). Hypochlorite Stress Assay for Phenotypic Analysis of the Halophilic Archaeon Haloferax volcanii Using an Improved Incubation Method and Growth Monitoring. Bio-protocol 12(22): e4557. DOI: 10.21769/BioProtoc.4557.
分类
微生物学 > 微生物生理学 > 胁迫反应
环境生物学
生物科学 > 微生物学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link














