- EN - English
- CN - 中文
In vitro Preparation of Homogenous Actin Filaments for Dynamic and Electrophoretic Light Scattering Measurements
用于动态和电泳光散射测量的均质肌动蛋白丝的体外制备
(*contributed equally to this work) 发布: 2022年11月20日第12卷第22期 DOI: 10.21769/BioProtoc.4553 浏览次数: 1180
评审: Giusy TornilloAnonymous reviewer(s)
Abstract
Actin filaments are essential for various biological activities in eukaryotic cellular processes. Available in vitro experimental data on these systems often lack details and information on sample preparation protocols and experimental techniques, leading to unreproducible results. Additionally, different experimental techniques and polymerization buffers provide different, sometimes contradictory results on the properties of these systems, making it substantially difficult to gather meaningful data and conclusive information from them. This article presents a robust, accurate, detailed polymerization protocol to prepare high-quality actin filament samples for light scattering experiments. It has been shown to provide unicity and consistency in preparing stable, dispersed, aggregates-free, homogenous actin filament samples that could benefit many other scientific research groups currently working in the field. To develop the protocol, we used conventional actin buffers in physiological conditions. However, it can easily be adapted to prepare samples using other buffers and biological fluids. This protocol yielded reproducible results on essential actin filament parameters such as the translational diffusion coefficient and electrophoretic mobility. Overall, suitable modifications of the proposed experimental method could generate accurate, reproducible light scattering results on other highly charged anionic filaments commonly found in biological cells (e.g., microtubules, DNAs, RNAs, or filamentous viruses).
Graphical abstract:
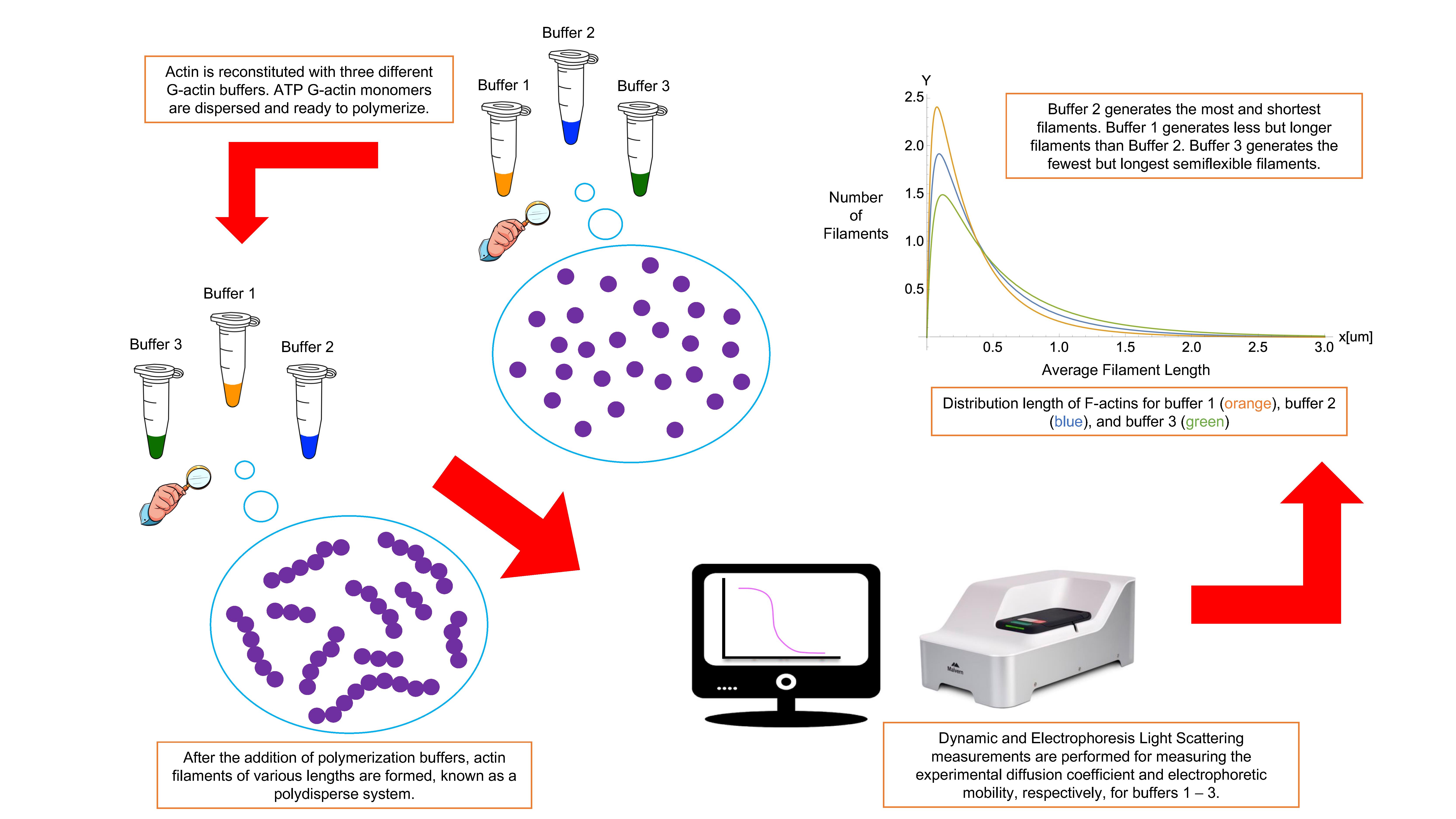
Background
Actin filaments (F-actin) are highly charged double-stranded semiflexible polyelectrolytes formed by the polymerization of G-actin subunits. Due to their hydrodynamic, mechanical, and electrostatic properties, these cytoskeleton filaments have the extraordinary ability to dynamically change conformations in response to alterations in G-actin concentration and type of crosslinker/binding proteins, as well as electrolyte concentration. These cytoskeleton properties are crucial for eukaryotic cells to achieve specific biological functions in different cellular compartments, which may vary depending on the cell type and location conditions. While a substantial amount of research has been done in the biochemistry and biophysics fields (Lanni and Ware, 1984; Janmey et al., 1986, 1994; Hou et al., 1990; Käs et al., 1996; Xu et al., 1998; Bonet et al., 2000; Niranjan et al., 2001; Y. H. Wang and Narayan, 2008; Crevenna et al., 2013; Del Rocio Cantero et al., 2020), the underlying principles and molecular mechanisms that support the hydrodynamic, electrostatic, and stability properties of these filaments remain elusive (Lanni and Ware, 1984; F. Wang et al., 1989; Hou et al., 1990; Käs et al., 1996; Bonet et al., 2000).
Modern dynamic light scattering (DLS) and electrophoretic light scattering (ELS) instruments are the most accurate, non-invasive, experimental techniques to characterize hydrodynamic and electrostatic properties of polydisperse charged biomolecules even at low concentrations and using small sample volumes. These instruments use advanced technology and multi-functional software to measure the hydrodynamic size, polydispersity, dispersion stability and aggregation, biomolecular charge, and zeta potential of particles and polymers immersed in aqueous biological environments. To assure high accuracy and reproducibility of the results, it is essential to use high-quality samples, accurate instruments, robust software, and optimized measurement protocols.
This article presents a complete, accurate, detailed polymerization protocol to prepare high-quality actin filament samples for light scattering experiments. Obtaining actin filament samples that are stable, dispersed, clean from aggregates and impurities, and homogenous could benefit many other scientific research groups in the field. We describe the polymerization procedure for three typical actin buffers in physiological conditions (Janmey et al., 1986, 1994; F. Wang et al., 1989). Nevertheless, this protocol can easily be adapted to prepare samples using other buffers and biological fluids. Additionally, we provide a unique methodology based on fundamental biostatistical tools (McDonald, 2009) and a measurement protocol with optimized configuration to perform DLS and ELS experiments on actin filament samples at critical G-actin concentrations. We measured actin filament’s translational diffusion coefficient and electrophoretic mobility using the same sample and experimental conditions for both DLS and ELS experiments to assure unicity and consistency in the results (Alva et al., 2022). A fitting approach was adequate to characterize other filaments’ essential properties, such as asymmetric length distribution, effective electric charge, hydrodynamic size, effective diameter, dispersion stability, aggregation, and semi-flexibility (Kroy and Frey, 1997; Tassieri et al., 2008; Alva et al., 2022). Additionally, the comparison of the light scattering results obtained for different polymerization buffers elucidated the impact of their chemical composition, reducing agents, pH values, and ionic strengths on the hydrodynamic, electrostatic, and stability properties of these actin structures. This kind of comparison and characterization provides a deeper understanding of how actin filaments behave in various cellular environments and conditions. Overall, suitable modifications of the proposed experimental method might allow other scientific groups to obtain accurate and reproducible light scattering results on other highly charged anionic polyelectrolyte filaments, commonly found in biological cells (e.g., microtubules, DNA, RNA, or filamentous viruses), having hydrodynamic, electrostatic, and stability properties resembling those characterizing actin filaments (Janmey et al., 2014).
Materials and Reagents
2 mL glass vial (Agilent, catalog number: 20097845)
Polypropylene centrifuge tube, 15 and 50 mL (Corning, catalog number: 430791)
Polypropylene microfuge tube, 11 × 38 mm, 1.5 mL (Beckman Coulter, catalog number: 9080511)
12 mm square polystyrene cell cuvettes (Malvern Panalytical, number: DTS0012)
2 mL cryogenic vials (Corning, catalog number: 30721070)
Business source stainless steel scissors (Fiskars, catalog number: 01-004250J)
Optifit pre-sterilized tips, 10–1,000 μL (Sartorius, catalog number: PR151159)
Optifit pre-sterilized tips, 5–350 μL (Sartorius, catalog number: PR149531)
Quartz spectrophotometer cell rectangular, stopper, 1 mm (Starna Cells, catalog number: 21Q1)
Actin protein (>99% pure): rabbit skeletal muscle; store at 4 °C (Cytoskeleton, catalog number: AKL99)
Water for molecular biology (Millipore, catalog number: H20MB1001)
Calcium chloride, dried, powder, 97% (CaCl2) (Alfa Aesar, catalog number: 10219230)
Magnesium chloride, anhydrous, 99% (MgCl2) (Alfa Aesar, catalog number: W07D102)
Potassium chloride (KCl) (VWR, BDH Chemicals, catalog number: 18J2256059)
Tris base (Fisher Bioreagents, catalog number: BP152-500)
Beta-mercaptoethanol, molecular biology grade (BME) (Millipore, catalog number: 3192024)
Adenosine triphosphate (ATP), 100 mM stock (Cytoskeleton, catalog number: BSA04)
DL-Dithiothreitol (DTT), molecular grade (Promega Corporation, catalog number: 0000383224)
Sodium hydroxide, pellets, 97%, A.C.S. reagent (NaOH) (Sigma-Aldrich, catalog number: 1310-73-2)
HCl volumetric standard, 0.1 N solution in water (Sigma-Aldrich, catalog number: 7647-01-0)
Precision red protein assay reagent (Cytoskeleton, catalog number: ADV02)
5 3/4” pipets (Fisherbrand, Fisher Scientific, catalog number: 13-678-20A)
70% v/v denatured ethanol solution (Fisher Bioreagents, catalog number: 216731)
Orion buffers pH 4.01, pH 7.00, and pH 10.00 (Thermo Scientific, catalog numbers: YX1, YW1, YX1)
Universal dip cell kit (Malvern Panalytical, catalog number: ZEN1002)
50 mM CaCl2 (see Recipes)
50 mM MgCl2 (see Recipes)
1.0 M KCl (see Recipes)
102.24 mM KCl (see Recipes)
G-actin buffer 1 (pH 7.80) (see Recipes)
G-actin buffer 2 (pH 7.66) (see Recipes)
G-actin buffer 3 (pH 8.23) (see Recipes)
Polymerization buffer 1 (pH 7.56) (see Recipes)
Polymerization buffer 2 (pH 7.64) (see Recipes)
Polymerization buffer 3 (pH 8.07) (see Recipes)
Electrolyte buffer 1 (pH 7.72), buffer 2 (pH 7.66), and buffer 3 (pH 8.06) (see Recipes)
Equipment
Analytical Explorer Pro-Scale (Ohaus Industrial Scales, model: PX84)
Pipette+ (Sartorius, Andrew Alliance Stand+)
Smart electronic pipettes: 5–350 μL, 10–1,000 μL, 5–10 mL (Sartorius, Andrew Alliance Stand+)
Vortex mixer with standard tube head, 120V (Corning LSE, The Lab Depot, Ref:6775)
Magnetic stirrer RT Basic-12 (Thermo Scientific, catalog number: 88880007)
Orion Star A211 pH meter, accuracy: ± 0.002 (Thermo Scientific, catalog number: X56954)
Allegra 64R benchtop centrifuge machine (Beckman Coulter, product number: 367585) with a F1202 Rotor, 30,000 rpm (Beckman Coulter, catalog number: 19U3138)
Zetasizer ULTRA, accuracy MW: ± 10% typical, temperature accuracy: 0.1 °C (Malvern Panalytical, model: ZSU5700)
Cary 100 UV-Vis spectrophotometer, accuracy: ~2% (Agilent Technologies, product number: 10069000)
Software
ZS Xplorer software (Malvern Panalytical, https://www.malvernpanalytical.com/en/products/product-range/zetasizer-range/zetasizer-advance-range/zetasizer-ultra)
Microsoft Word 2021 (Microsoft, https://www.microsoft.com/)
Cary WinUV Kinetics (Agilent Technologies, https://www.bioprocessonline.com/doc/cary-winuv-software-0001)
Cary WinUV Scan (Agilent Technologies, https://www.bioprocessonline.com/doc/cary-winuv-software-0001)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Alva, E., George, A., Brancaleon, L. and Marucho, M. (2022). In vitro Preparation of Homogenous Actin Filaments for Dynamic and Electrophoretic Light Scattering Measurements. Bio-protocol 12(22): e4553. DOI: 10.21769/BioProtoc.4553.
分类
神经科学 > 神经解剖学和神经环路
生物科学 > 生物技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
提问指南
+ 问题描述
写下详细的问题描述,包括所有有助于他人回答您问题的信息(例如实验过程、条件和相关图像等)。
Share
Bluesky
X
Copy link









