- EN - English
- CN - 中文
In vitro Assay to Evaluate Cation Transport of Ionophores
评估离子载体阳离子转运的体外检测方法
发布: 2022年11月20日第12卷第22期 DOI: 10.21769/BioProtoc.4552 浏览次数: 2262
评审: David PaulJohn P PhelanAnonymous reviewer(s)
Abstract
Ion homeostasis is a fundamental regulator of cellular processes and depends upon lipid membranes, which function as ion permeability barriers. Ionophores facilitate ion transport across cell membranes and offer a way to manipulate cellular ion composition. Here, we describe a calcein quenching assay based on large unilamellar vesicles that we used to evaluate divalent cation transport of the ionophore 4-Br-A23187. This assay can be used to study metal transport by ionophores and membrane proteins, under well-defined conditions.
Graphical abstract:
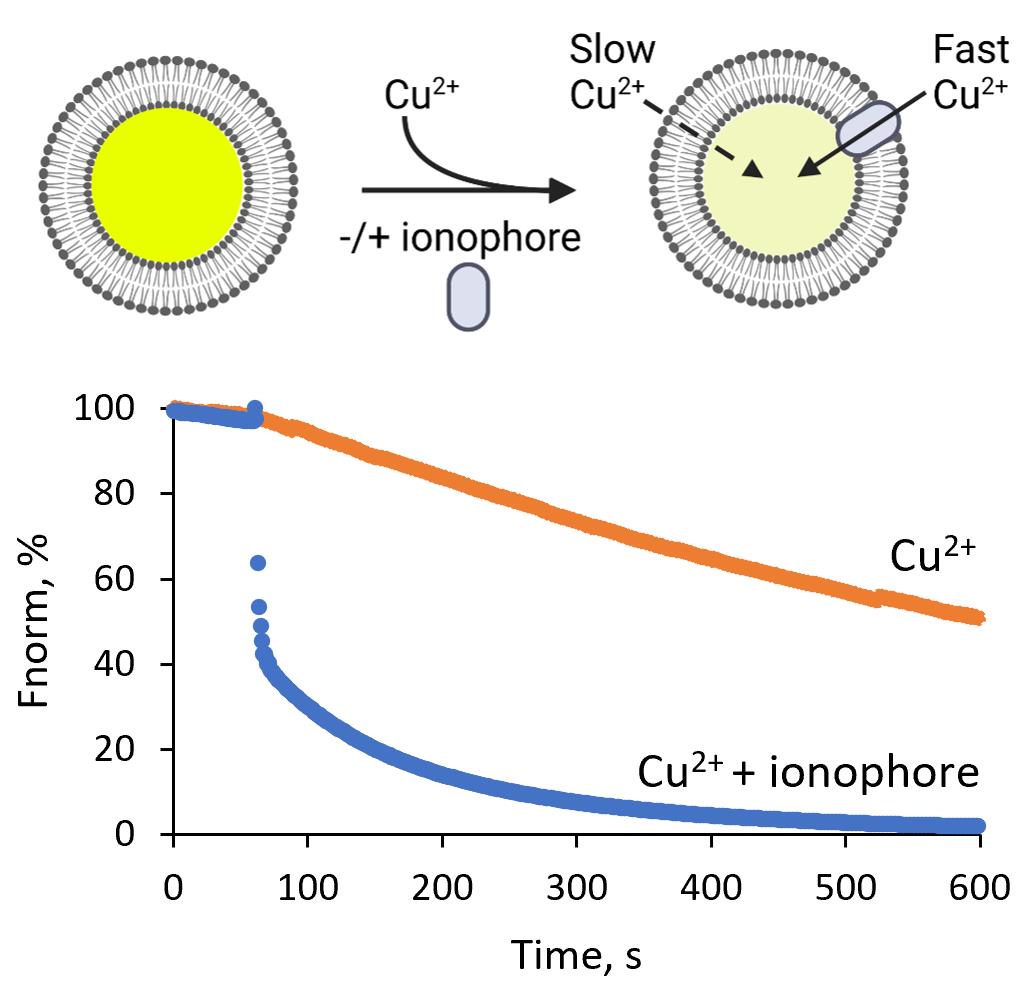
Background
Ion homeostasis is a fundamental requirement for all living cells, as it controls diverse biological processes, including cell signaling, maintenance of cell volume and osmotic pressure, and regulation of cellular pH. Ions also act as enzyme cofactors, providing force through concentration gradients, and serve as corepressors for protein regulators orchestrating, for instance, the oxidative stress response. Ion homeostasis in cells depends upon lipid membranes, which function as ion permeability barriers. This allows cells to maintain highly asymmetric concentrations of cations and anions across both plasma membranes and intracellular organelles. Major ionic gradients across the plasma membrane include sodium (Na+), potassium (K+), calcium (Ca2+), and chloride (Cl-). Ionophores, which transport ions across biological membranes, offer a way to manipulate cellular ion composition. Examples of commonly used ionophores include valinomycin, which transports potassium (K+) (Su et al., 2019), or calcimycin (A23187) and ionomycin, which transport zinc (Zn2+), manganese (Mn2+), and Ca2+ (Erdahl et al., 1994, 1995, 1996), or 4-bromocalcimycin (4Br-A23187), which is highly selective for the transport of Zn2+ and Mn2+, compared to Ca2+ (Erdahl et al., 1996). Ion transport and specificity of ionophores are typically characterized in vitro, in model systems comprising model membranes and a single ion species available for transport. A common cell membrane model is based on large unilamellar vesicles (LUVs) formed from one lipid type or lipid mixtures of different compositional complexity. The vesicles are typically prepared from a lipid solution in a volatile organic solvent. A thin lipid film on the inside of a glass flask is formed by slow evaporation of the organic solvent. The subsequent hydration of the lipid film results in the formation of large multilamellar vesicles, which are converted to unilamellar vesicles of defined size by a freeze-thaw and extrusion method (Olson et al., 1979; Hope et al., 1986; Mayer et al., 1986; Kunding et al., 2008). This approach has been used to encapsulate radioactive ions (e.g., 22Na+, 45Ca2+, and the K+ mimic 86Rb+) inside the liposomes to study their efflux upon dilution of the liposomes into an isotope-free solution (e.g., Hamilton and Kaler, 1987 and 1990). A drawback to this procedure is the inconvenience associated with handling radioactive reagents. Alternative assays are based on the encapsulation of water-soluble ion-sensitive dyes into the vesicle. Ion permeability is then monitored by fluorescence spectroscopy. The protocol presented here utilizes calcein as a fluorescent dye, which can be quenched by Cu2+, Ni2+, Co2+, and Fe3+ at neutral pH (Černiauskas et al., 2017; Picard et al., 2000), and was recently used by us to reveal 4-Br-A23187 as a potent copper ionophore (Senges et al., 2022). Given the broad range of ions that can quench calcein fluorescence, the assay should be useful for determining the selectivity and mechanisms of ionophore-mediated cation transport. Furthermore, the impact of ionophores on membrane integrity can be tested using a calcein leakage assay by loading the vesicles with a self-quenching concentration of calcein (> 750 µM), as described previously (Maherani et al., 2013; Dutta et al., 2020; Bae et al., 2021; Senges et al., 2022). This versatile protocol can be fitted to other ion-sensitive dyes, although encapsulation efficiency should be tested for each dye. The assay can also be adapted to study metal transport by reconstituted membrane proteins (Billesbølle et al., 2020).
Materials and Reagents
Ice bucket (e.g., Magic Touch 2TM ice bucket with lid; Sigma-Aldrich, catalog number: BAM168072002)
Glass bead, 3 mm (Merck, catalog number: 104015)
Microcentrifuge tubes of 1.5 mL capacity (SARSTEDT AG & Co. KG, catalog number: 72.690.001)
Polyethersulfone membrane with a pore size of 0.2 μm (Filtropur, SARSTEDT AG & Co. KG, catalog number: 83.1826.001)
Polypropylene tubes of 15 mL and 50 mL capacity (e.g., Falcon tubes, SARSTEDT AG & Co. KG, catalog numbers: 62.554.502 and 62.547.254)
Round bottom glass tubes (16 × 150 mm, Carl Roth, catalog number: NY90.1)
Magnetic bars ROTILABO® Micro (diameter 2 mm, length 5 mm, Carl Roth, catalog number: 0955.2)
Syringe filter, Filtropur S, membrane: Polyethersulfone (PES), filtration surface: 6.2 cm2, pore size: 0.2 µm, for sterile filtration (Sarstedt, catalog number: 83.1826.001)
Syringe sterile dimethyl sulfoxide (DMSO; Thermo Scientific, catalog number 036480.AP)
1,2-dioleoyl-sn-glycerophosphatidylcholine (DOPC; Avanti Polar Lipids, catalog number: 850375C)
Aluminum foil
Calcein (Merck, catalog number: 1461-15-0)
Calcium chloride (Grüssing, catalog nummer: 102331000U)
Chloroform, ethanol-stabilized and certified for absence of phosgene and HCl (VWR, catalog number: 22711.290)
Deionized water
HEPES (Carl Roth, catalog number: 6763.3)
Liquid nitrogen
Methanol (VWR, catalog number: 20834.291)
N2 gas (ALPHAGAZ 1 N2, 99.999%, Air Liquide, Germany)
Potassium chloride (Sigma-Aldrich, catalog number: 7447-40-7)
Potassium hydroxide (Fisher Scientific, catalog number: P250-1)
SephadexTM G-50 fine (GE Healthcare Bio-Sciences AB, catalog number: 17-0042-01)
Triton X®-100, extra pure (Carl Roth, catalog number: 3051.3)
4-Br-A23187 (Cfm Oskar Tropitzsch, Marktredwitz, catalog number: 76455-48-6)
Buffer A (200 mL) (see Recipes)
Calcein stock in buffer A (16.6 mM, 1 mL) (see Recipes)
CuCl2 stock (2 mM) (see Recipes)
G50-Gel in buffer A (see Recipes)
HEPES, pH 7.4 (0.5 M, 200 mL) (see Recipes)
Ionophore stock (6 mM) (see Recipes)
KCl (0.5 M, 200 mL) (see Recipes)
KOH (1 M) (see Recipes)
Lipid stock in chloroform (see Recipes)
Loading buffer (1 mL) (see Recipes)
Triton X-100 solution (20% w/v) (see Recipes)
Equipment
Forceps (e.g., VWR, catalog number: 232-0032)
Analytical balance (e.g., Sartorius Entris-I II, 220 g/0.1 mg; Buch Holm, catalog number: 4669128)
Centrifuge with rotor for 15 mL polypropylene tubes (e.g., Eppendorf 5810 R)
Flow cabinet to work with organic solvents
Fluorometer (PTI QuantaMaster 800 fluorometers) with integrated FelixGX software, equipped with single cuvette Peltier K-155-C temperature control and magnetic stirrer (Horiba)
Freezer (-20 °C)
Hamilton 700 Series Syringes of 10 µL, 100 µL, 250 µL, 500 µL, and 1,000 µL (Hamilton® syringe, 700 and 1000 Series)
Macro polystyrene cuvettes (SARSTEDT AG & Co. KG, Germany, maximum 4.5 mL volume, catalog number: 67.745)
Magnets 5 × 2 mm (Merck, catalog number: Z328839)
Mini-Extruder Set (Avanti Inc., catalog number: 610023)
1 mL gas-tight syringes (Avanti no: 610017)
10 mm filter supports (Avanti no: 610014)
19 mm Nucleopore tracketch membrane with a pore size of 0.2 μm (Schleicher & Schuell)
pH-meter (pH-Meter 761 Calimatic, Knick)
Pipettes P20, P200, P1000 (GILSON®, catalog numbers: FD10001, FD10005, and FD10006)
Pipette tips 2 µL, 20 µL, 200 µL, and 1,000 µL (SARSTEDT AG & Co. KG, catalog numbers: 70.1130.212, 70.3021, 70.760.002, and 70.3050.020)
Refrigerator (4 °C)
Scissors
Rotavapor® R-100 Evaporator with I-100 Controller and V-100 vacuum pump (Flawil, Switzerland)
Vortexer (Vortex Genie 2TM, BENDER & HOBEIN AG, Switzerland)
Water bath (e.g., WPE45 Memmert, Germany)
Software
FelixGX software for the control of the PTI QuantaMaster 8000 fluorometer and accessories
Microsoft Excel for Microsoft 365 MSO (Version 2205)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Uzun, H. D., Vázquez-Hernández, M., Bandow, J. E. and Pomorski, T. G. (2022). In vitro Assay to Evaluate Cation Transport of Ionophores. Bio-protocol 12(22): e4552. DOI: 10.21769/BioProtoc.4552.
分类
药物发现 > 药物筛选
生物化学 > 其它化合物 > 离子
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













