- EN - English
- CN - 中文
X-ray Crystallography: Seeding Technique with Cytochrome P450 Reductase
X射线晶体学:细胞色素P450还原酶加晶种技术
发布: 2022年11月05日第12卷第21期 DOI: 10.21769/BioProtoc.4546 浏览次数: 2275
评审: Joana Alexandra Costa ReisLaura AlviginiAnonymous reviewer(s)
Abstract
Cytochrome P450 reductase (CPR) is a multi-domain protein that acts as a redox partner of cytochrome P450s. The CPR contains a flavin adenine dinucleotide (FAD)–binding domain, a flavin mononucleotide (FMN)-binding domain, and a connecting domain. To achieve catalytic events, the FMN-binding domain needs to move relative to the FAD-binding domain, and this high flexibility complicates structural determination in high-resolution by X-ray crystallography. Here, we demonstrate a seeding technique of sorghum CPR crystals for resolution improvement, which can be applied to other poorly diffracting protein crystals. Protein expression is completed using an E. coli cell line with a high protein yield and purified using chromatography techniques. Crystals are screened using an automated 96-well plating robot. Poorly diffracting crystals are originally grown using a hanging drop method from successful trials observed in sitting drops. A macro seeding technique is applied by transferring crystal clusters to fresh conditions without nucleation to increase crystal size. Prior to diffraction, a dehydration technique is applied by serial transfer to higher precipitant concentrations. Thus, an increase in resolution by 7 Å is achieved by limiting the inopportune effects of the flexibility inherent to the domains of CPR, and secondary structures of SbCPR2c are observed.
Graphical abstract:
Background
Cytochrome P450 reductase (CPR, EC 1.6.2.4) is located at the cytosol side of the endoplasmic reticulum membrane in eukaryotic cells, acting as the electron donor to cytochrome P450s and other heme proteins (Phillips and Langdon, 1962). It has been known that there are one to three CPR genes in most vascular plants (Jensen and Møller, 2010). The CPR contains a flavin adenine dinucleotide (FAD)/NADPH-binding domain, a flavin mononucleotide (FMN)-binding domain, and a connecting (or linker) domain with a flexible hinge region that links these two domains (Munro et al., 2001; Wang et al., 1997). We have reported on the structural flexibility of sorghum CPR isoforms (Zhang et al., 2022b) and the rapid interdomain opening and closing motion that resulted in fast interdomain electron transfer in SbCPR (Zhang et al., 2022a). Especially in the SbCPR2c isoform, which was crystallized in full-length, the FMN-binding domain was not resolved due to the aforementioned flexibility. Such flexibility causes uncertainty of the residue atomic coordinates and dihedral angles of the protein crystal, which leads to local disordered structure and overall low-resolution diffraction.
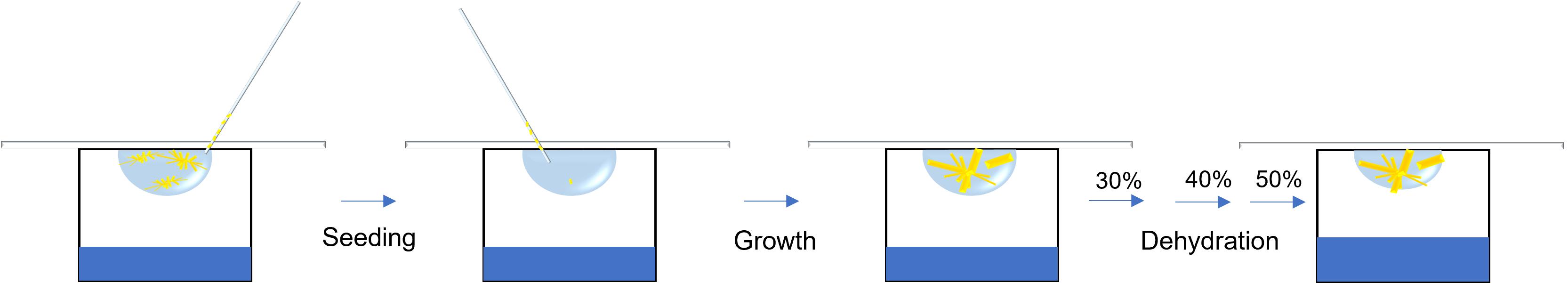
Crystallization of diffraction quality crystals is the largest bottleneck in protein structure determination (Holcomb et al., 2017). These difficulties have been overcome through the use of various techniques. Seeding uses previously nucleated crystals to initiate the growth of larger crystals in a fresh drop where protein concentration has not yet been depleted by the many nucleation sites of the small crystals (Rhodes, 2006). Micro streaking operates by running a fiber over an existing crystal—often a whisker from an animal—through a fresh drop to increase growth of the nucleated particles in a supersaturated drop (Stura and Wilson, 1991). In contrast, a macro seeding technique utilizes larger crystals, and thus requires more precision as a chosen crystal is washed and transferred from its original drop to a new mother liquor. This method tends to be more meticulous and can fracture the original crystal, causing smaller fragments to be placed into the new drop, which induces micro seeding (Zhu et al., 2005). Seeding techniques have allowed for growth of larger crystals and increased resolution of diffraction; cellobiohydrolase II (CBHII) crystallization was first achieved by using seeding to avoid formation of microcrystals and diffraction greater than 2.0 Å (Bergfors et al., 1989). High mosaicity and cracking/sliding of the lattice caused by impurities have been overcome by using seeding to reduce crystal deficits and improve the resolution of crystals (Caylor et al., 1999). Another method for improving crystal lattice packing is the dehydration of the crystalline to reduce water contents and to consequently confer tighter packing (Timasheff, 1995). Dehydration can be accomplished via exposure to the atmosphere or via serial dilution into higher cryoprotectant-containing solutions (Heras and Martin, 2005). Crystal dehydration has been one of the most successful post crystallization treatments in increasing resolution. Notably, the resolution of disulfide bond isomerase was improved from 7 to 2.6 Å and from 12 to 2.6 Å in E. coli YbgL (Abergel, 2004; Haebel et al., 2001).
We incorporated a macromolecule seeding technique—additive solution optimization—together with dehydration to improve the resolution of the SbCPR2c crystal structure from approximately 12 to 4.5 Å. This enabled the determination of the secondary structure for SbCPR2c. It is worth noting, however, that crystal growth is a very tedious process with repeatability differing in trials due to miniscule changes of parameters and the complexity of the system. Despite the aforementioned difficulties, our protocol of seeding and dehydration could be widely applied to resolve the resolution problems to a variety of crystals hindered in resolution by their dynamic disorder.
Materials and Reagents
MRC 2-well crystallization plate (Swissci, Hampton Research, catalog number: HR3-082)
24-well XRL plate (Molecular Dimensions, catalog number: MD3-11)
Additive screen (Hampton Research, catalog number: HR2-428)
Microscope cover glass (Fisher Scientific, Fisherbrand, catalog number: 19803)
Glass capillary (Charles Super Company, catalog number: 02-SG)
Amicon 8050 ultrafiltration cell (Amicon, catalog number: UFSC05001)
30 kDa ultrafiltration discs (EMD Millipore Corporation, Biomax, catalog number: PBTK04310)
Escherichia coli Rosetta 2 (DE3) competent cells (Sigma-Aldrich, Millipore Sigma, catalog number: 71402)
pET-30a(+) vector (Sigma-Aldrich, Millipore Sigma, catalog number: 69909)
Sorghum bicolor CPR2c gene (Sobic.006G245400) (synthesized by Genscript)
Kanamycin (IBI Scientific, catalog number: IB02120)
IPTG (GoldBio, catalog number:I2481C100)
NADP+ (Alfa Aesar, catalog number: T23B015)
PEG 3350 (Sigma-Aldrich, catalog number: P4338)
Nickel-NTA resin (G-biosciences, catalog number: 786-940)
Index crystal screen kit (Hampton Research, catalog number: HR2-144)
Luria-Bertani (LB) medium (see Recipes)
Lysis/wash buffer (see Recipes)
Elution buffer (see Recipes)
Hydroxyapatite column buffer A (see Recipes)
Hydroxyapatite column buffer B (see Recipes)
Final buffer (see Recipes)
Crystallization buffer (crystal screen F12) (see Recipes)
Equipment
Crystal Phoenix (Art Robbins Instruments)
ÄKTA pure (GE Healthcare, ÄKTA pure, catalog number: 29014834)
Model 450 sonicator (Branson Ultrasonics, catalog number: 22-309783)
Software
HKL2000 (https://hkl-xray.com/hkl-2000)
Phenix (https://phenix-online.org/)
Coot (https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Zhang, B., Lewis, J. A., Hazra, R. and Kang, C. (2022). X-ray Crystallography: Seeding Technique with Cytochrome P450 Reductase. Bio-protocol 12(21): e4546. DOI: 10.21769/BioProtoc.4546.
- Zhang, B., Munske, G. R., Timokhin, V. I., Ralph, J., Davydov, D. R., Vermerris, W., Sattler, S. E. and Kang, C. (2022b). Functional and structural insight into the flexibility of cytochrome P450 reductases from Sorghum bicolor and its implications for lignin composition. J Biol Chem 298(4): 101761.
分类
植物科学 > 植物生物化学 > 蛋白质 > 结构
生物物理学 >
生物化学 > 蛋白质 > 结构
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link