- EN - English
- CN - 中文
In situ cryo-FIB/SEM Specimen Preparation Using the Waffle Method
用华夫饼法原位制备冷冻双束电镜FIB/SEM的样品
(*contributed equally to this work) 发布: 2022年11月05日第12卷第21期 DOI: 10.21769/BioProtoc.4544 浏览次数: 5914
评审: Manjula MummadisettiVignesh KasinathAnonymous reviewer(s)
Abstract
Cryo-focused ion beam (FIB) milling of vitrified specimens is emerging as a powerful method for in situ specimen preparation. It allows for the preservation of native and near-native conditions in cells, and can reveal the molecular structure of protein complexes when combined with cryo-electron tomography (cryo-ET) and sub-tomogram averaging. Cryo-FIB milling is often performed on plunge-frozen specimens of limited thickness. However, this approach may have several disadvantages, including low throughput for cells that are small, or at low concentration, or poorly distributed across accessible areas of the grid, as well as for samples that may adopt a preferred orientation. Here, we present a detailed description of the “Waffle Method” protocol for vitrifying thick specimens followed by a semi-automated milling procedure using the Thermo Fisher Scientific (TFS) Aquilos 2 cryo-FIB/scanning electron microscope (SEM) instrument and AutoTEM Cryo software to produce cryo-lamellae. With this protocol, cryo-lamellae may be generated from specimens, such as microsporidia spores, yeast, bacteria, and mammalian cells, as well as purified proteins and protein complexes. An experienced lab can perform the entire protocol presented here within an 8-hour working day, resulting in two to three cryo-lamellae with target thicknesses of 100–200 nm and dimensions of approximately 12 μm width and 15–20 μm length. For cryo-FIB/SEMs with particularly low-contamination chambers, the protocol can be extended to overnight milling, resulting in up to 16 cryo-lamellae in 24 h.
Graphical abstract:
Background
Studying biological systems in close-to-native or in situ states opens wide frontiers towards precise contextual understandings of the mechanistic behaviors of molecular machines. In cryo-electron tomography (cryo-ET) pipelines, the sample is vitrified, which 1) captures and preserves biological processes in their close-to-native state, 2) maintains sample hydration, and 3) protects it from the vacuum of the electron microscope (Saibil, 2022). Vitrification is commonly followed by sample thinning with cryo-focused ion beam (cryo-FIB) milling (Marko et al., 2007) before cryo-ET data acquisition, providing the highest resolution in situ three dimensional (3D) views into the structures of individual protein complexes and cell parts in native contexts (Wang et al., 2022). Cryo-ET also allows for the assessment of the overall morphology of more complex biological systems, including cellular organelles, whole cells, intact organisms, and tissues (Bauerlein and Baumeister, 2021). However, vitrification with common plunge-freezing approaches is limited to samples up to ~5–10 μm thick. For thicker samples, a high-pressure freezer (HPF) must be used, which vitrifies specimens up to at least 60 μm thick (Harapin et al., 2015). HPF vitrification has been successfully applied to multicellular biological samples (Harapin et al., 2015), and its applicability has been extended with the waffle method (Kelley et al., 2022). The waffle method allows for 1) vitrification of samples with thicknesses of tens of microns, 2) elimination of preferred orientation issues, 3) production of large (between 10 μm × 10 μm and 70 μm × 25 μm in x,y) and specimen-dense cryo-lamellae, and 4) an increase in cryo-FIB lamellae production throughput.
Specimens vitrified with an HPF cannot be directly imaged by cryo-ET and need to be thinned down to <300 nm to be transparent enough to the electron beam. During the last decade, cryo-FIB has gained substantial traction for thinning vitrified biological specimens, overtaking the promising but technologically challenging method of cryo-ultramicrotomy (McDowall et al., 1983; Marko et al., 2007). Until several years ago, HPF vitrification of large (10+ μm thick) specimens followed by cryo-FIB/SEM and cryo-ET has typically been demonstrated on multicellular C. elegans organisms (Harapin et al., 2015; Mahamid et al., 2015; Schaffer et al., 2019). Direct cryo-FIB milling of these samples requires a significant amount of continuous milling (>1 day) and has a low success rate. To increase the throughput, Mahamid et al. (2015) and Schaffer et al. (2019) developed a cryo-liftout (cryo-LO) approach, where a section of the specimen is extracted with a micro-manipulator and transferred to a special half-moon grid, for further thinning. Cryo-LO allows for region of interest (ROI) extraction, and decreases milling times per single lamella of ~150 nm thickness and a suitable imaging area of ~8 μm × 7 μm to ~10 h (Schaffer et al., 2019); however, it requires specialized milling equipment and expertise.
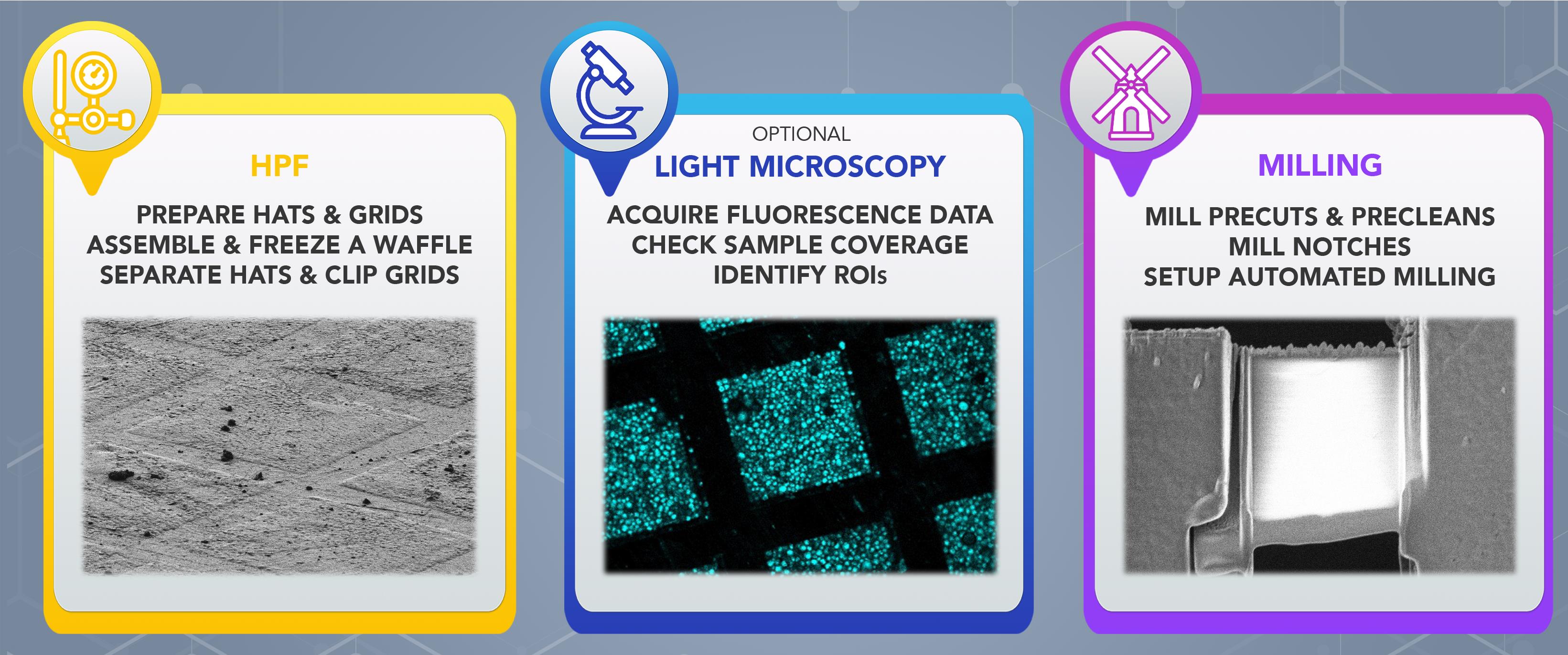
To address these issues, we developed the waffle method, as described in Kelley et al. (2022). Here, we present a detailed protocol to support the waffle method, including sample vitrification (Procedure A), and waffle sample milling (Procedure B), that is applicable to a variety of biological samples. Samples prepared with Procedure A are generally tens of microns thick. Milling of these waffle samples does not require specialized instrumentation and can be performed manually or semi-automatically. Procedure B describes semi-automated milling, where milling takes ~2 h per lamellae site, with target thickness of 150–200 nm (as defined by the AutoTEM Cryo software), and approximate dimensions of 12 μm in width × 15–20 μm in length. Procedure B utilizes the MAPS software for defining ROIs and lamellae sites, then TFS AutoTEM Cryo automates the majority of the waffle milling process similarly to freely-available milling software packages (Buckley et al., 2020; Klumpe et al., 2021). Waffle lamellae are typically larger in cross-sectional area than lamellae generated with conventional plunge-frozen cryo-FIB-milling, and may be produced at a higher rate (~2,800 μm2 per 24 h for waffle lamellae versus ~600 μm2 per 24 h for conventional lamellae; see Notes for calculations).
To increase the quality and efficiency of the protocol, and to ensure that the resulting cryo-ET tomograms contain objects of interest, ROI localization is optionally done by correlating fluorescent images of the sample before or after freezing. Cryo-fluorescent light microscopy (cryo-FLM) is used to illuminate fluorescently-tagged objects in the vitrified sample and may be performed with a stand-alone cryo-FLM, or with an FLM integrated inside the milling instrument (iFLM, Gorelick et al., 2019). Cryo-FLM can be done before (as screening), during (for FIB-milling navigation), and after FIB-milling (for guiding data collection) (Watanabe et al., 2020; Gupta et al., 2021). However, it is important to account for autofluorescence for certain samples (Carter et al., 2018). In addition to fluorescence-based approaches, two other methods for localizing or obtaining statistical information of objects of interest in situ exist, but fall outside the scope of this protocol: samples with EM-tags for localization in cryo-ET data (Silvester et al., 2021; Tan et al., 2021), and structural mass spectrometry (Klykov et al., 2022).
Materials and Reagents
Figure 1 shows several of the required items listed below together with common laboratory equipment.
200-mesh cryo-EM grids (e.g., SPI Supplies, catalog number: 4220C-CF)
Note: Grids need to be coated with an extra ~20 nm thick carbon layer. While most commonly used grid material can be utilized, we typically use 200-mesh copper grids. Larger and smaller mesh sizes will decrease and increase lamellae dimensions, respectively. Grid foil type is not important, as it will be milled through.
Gridboxes (e.g., SubAngstrom, catalog number: GBV01)
Freezer planchettes (hats) type “B” (e.g., Ted Pella, catalog number: 39201)
SEM pin stub (e.g., Ted Pella, catalog number: 16261)
Abrasive sandpaper with grits 1,200, 7,000, 15,000, or similar (e.g., ADVcer, ADV_Sandpaper_Sheets_S01_5_10BUGY)
HPF holder (e.g., Technotrade International, catalog number: 290-1)
POL Metallpflege metal-polish (discontinued, substituted by, e.g., Ted Pella, catalog number: 892)
Filter papers (e.g., Whatman, catalog number: 1004090)
Kimwipe tissues (e.g., Kimtech, catalog number: 34155)
C-clip ring (Thermo Fisher Scientific, catalog number: 1036173)
Cryo-FIB AutoGrid (Thermo Fisher Scientific, catalog number: 1205101)
Yeast, bacteria, mammalian cells, proteins, or protein complexes
Concentrating supplies
For cells or organisms, Conical Sterile Polypropylene Centrifuge Tubes (e.g., Thermo Fisher Scientific, catalog number: 339652)
For purified proteins and protein complexes, protein concentrators with MWCO based on the protein size (e.g., Thermo Fisher Scientific, catalog number: 88513)
Respective cell culture or yeast media
Industrial grade dry nitrogen tank
Industrial grade liquid nitrogen tank
1-Hexadecene (e.g., Millipore Sigma, catalog number: 822064)
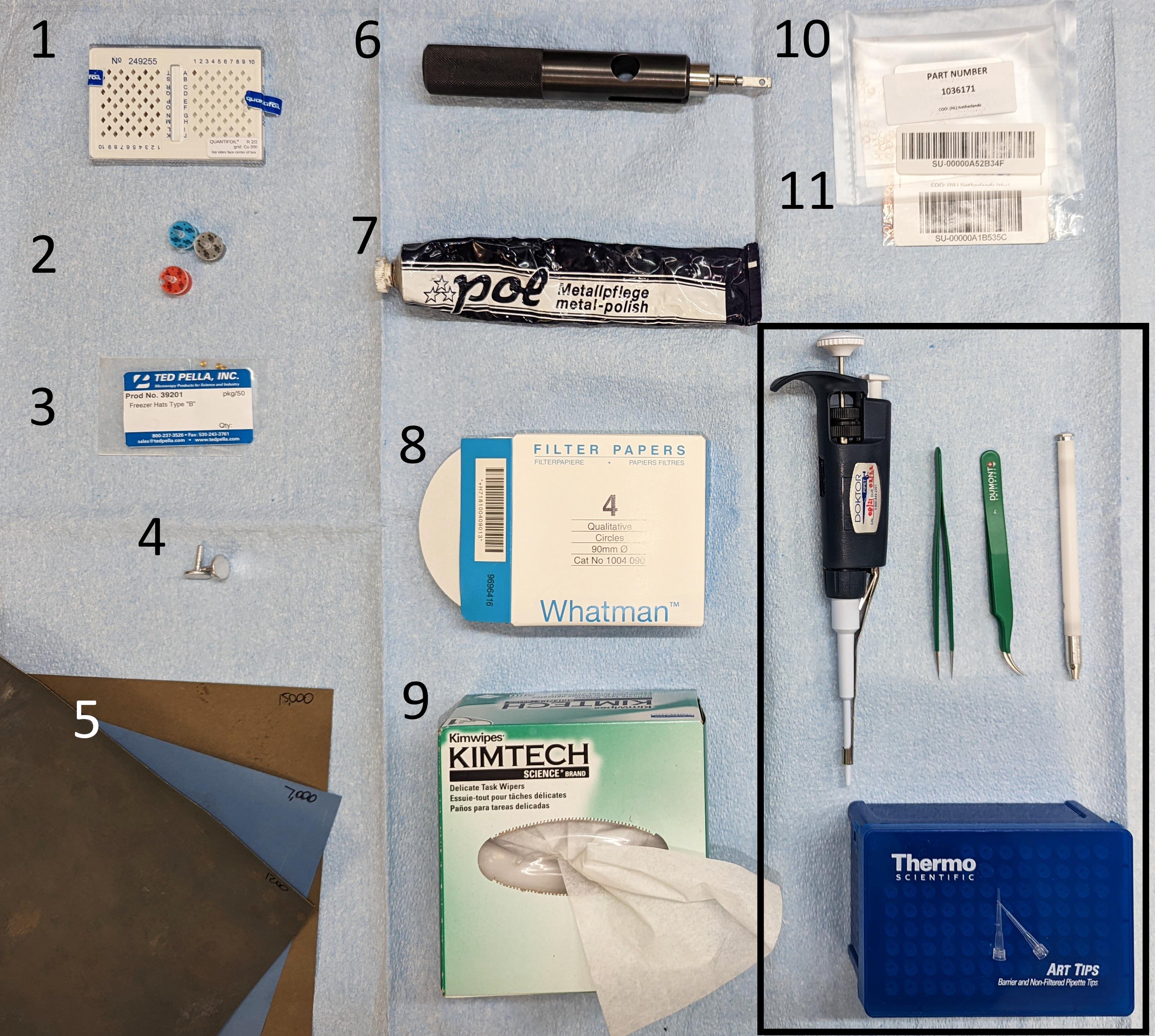
Figure 1. Waffle preparation instrumentation. The items from the Materials and Reagents section are shown with their corresponding numbering. For the waffle assembly, please refer to the original waffle paper by Kelley et al. (2022). Other common cryo-EM laboratory equipment included for completeness (black box on the bottom-right): P10 pipette with corresponding tips, fine-point tweezers, and AutoGrid box opening tool.
Equipment
Personal protective equipment for work with liquid nitrogen (e.g., gloves, eye protection, oxygen monitors)
Stereoscope (e.g., Zeiss, catalog number: 455053-0000-000)
Clipping station and clipping tools (e.g., Thermo Fisher Scientific, catalog number: 1000068)
Pipettors and tips (10 μL, 200 μL, 1,000 μL)
Centrifuge (e.g., Thermo Fisher Scientific, catalog number: 75004240)
Tweezers (e.g., Ted Pella, catalog number: 5220)
AutoGrid tweezers (e.g., Ted Pella, catalog number: 47000-600)
Grid transfer dewar (e.g., Thomas Scientific, catalog number: 5028M40)
Liquid Nitrogen dewar with 4 L capacity
Liquid Nitrogen dewar or puck system for grid storage (e.g., SubAngstrom, catalog number: GSS02-E)
Note: In our case, storing lamellae separated from other samples reduces contamination.
CCU-010 HV high vacuum coater (e.g., Safematic, catalog number: 100001)
CT-010 carbon fibre evaporation head module (e.g., Safematic, catalog number: 100003)
Plasma Cleaner (e.g., Gatan Inc., Gatan Solarus II Model 955)
Wohlwend Compact 01 HPF or comparable (M. Wohlwend GmbH)
Cryo-FIB/SEM dual beam microscope, including relevant grid shuttles (Thermo Fisher Scientific, Aquilos 2)
Note: The standard Aquilos 2 shuttle has a 45° pretilt. We found that the 27° pretilt shuttle supplied with the TFS iFLM module is more convenient for performing precleans (Procedure B, Step 30) because the pretilt allows for higher milling angles without lowering the stage.
Anti-contamination cryo-shield/cryo-shutter system (e.g., Delmic CERES Ice Shield, catalog number: DM-2707-999-0003-1)
Note: Such a low-contamination setup is optional. We found it useful in terms of reducing lamellae contamination during automated milling and allowing for automated overnight milling.
Software
FIB-milling software:
Microscope operating platform. Instruments from TFS are typically operated with xT. Here, we used xT v. 20.1.1.
Software for defining ROIs and aligning fluorescent signals, when applicable. Here we used MAPS v. 3.16-3.17.
Software for milling automation. Here we used AutoTEM Cryo v. 2.2.
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Klykov, O., Bobe, D., Paraan, M., Johnston, J. D., Potter, C. S., Carragher, B., Kopylov, M. and Noble, A. J. (2022). In situ cryo-FIB/SEM Specimen Preparation Using the Waffle Method. Bio-protocol 12(21): e4544. DOI: 10.21769/BioProtoc.4544.
分类
生物物理学 > 电子冷冻断层扫描
细胞生物学 > 细胞结构
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










