- EN - English
- CN - 中文
Fluorescent Binding Protein Sensors for Detection and Quantification of Biochemicals, Metabolites, and Natural Products
用于检测和量化生化物质、代谢物和天然产物的荧光结合蛋白传感器
发布: 2022年11月20日第12卷第22期 DOI: 10.21769/BioProtoc.4543 浏览次数: 2892
评审: Chiara AmbrogioAnonymous reviewer(s)
Abstract
Membrane transporters and soluble binding proteins recognize particular nutrients, metabolites, vitamins, or ligands. By modifying genetically engineered single cysteine residues near the active sites of such proteins with extrinsic maleimide fluorophores, the approaches that we report create sensitive fluorescent sensors that detect, quantify, and monitor molecules that are relevant to the biochemistry, physiology, microbiology, and clinical properties of pro- and eukaryotic organisms.
Graphical abstract:
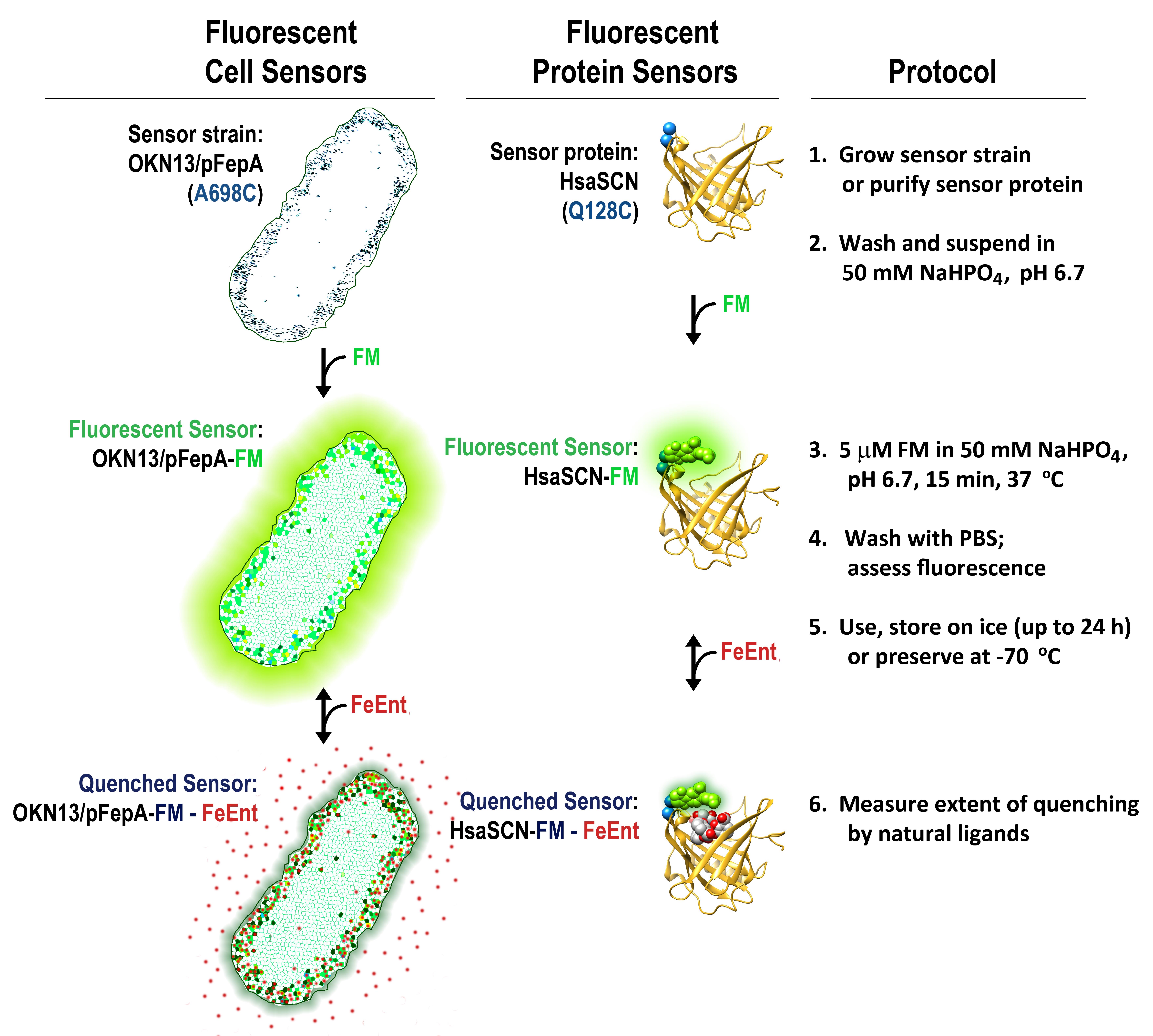
Background
The covalent modification of proteins to investigate their biochemical properties (Means and Feeney, 1971) was initially limited by the often uncertain location of amino acid side chains in protein tertiary structure, and the variable reactivity of different side chains with extrinsic chemical probes. However, the combination of protein sequence information, crystallographic depictions of protein folding, site-directed mutagenesis, and specific alkylation chemistry allowed high yield, often stoichiometric labeling of genetically engineered cysteine (Cys) sulfhydryls at strategic sites in proteins. Cys sulfhydryls are selectively modified by reagents like methanthiosulfonates, maleimides, and iodoacetamides (Means and Feeney, 1971). When combined with site-directed spin labeling experiments and electron paramagnetic resonance (EPR) spectroscopy, this approach provided information about the structure and function of membrane proteins (Altenbach et al., 1989, 1990). In addition, while exploring the properties of the major facilitator lactose permease, LacY, Kaback and colleagues adapted the method to site-directed fluorescent labeling (Nie et al., 2007; Smirnova et al., 2008). However, LacY is a Gram-negative bacterial inner membrane (IM) protein that is shielded by the barrier properties of the outer membrane [OM; Nikaido, 2003; Nikaido and Vaara, 1985] and hence recalcitrant to labeling in vivo. Our protocol focuses on fluorescent modification of engineered Cys residues on the external loops of Gram-negative bacterial OM proteins (OMPs), to transform them into sensors. This methodology enables labeling of living cells, which has many advantages, including facile preparation, high sensitivity, biochemical durability, multifunctionality, and convenient storage (Nairn et al., 2017; Chakravorty et al., 2019; Kumar et al., 2022). We also genetically engineered, purified, and fluorescently labeled binding proteins [e.g., human siderocalin (HsaSCN)] for use as soluble sensors. These manipulations transform both types of proteins into sensitive, quantitative biosensors that detect the presence of biochemicals, metabolites, natural products, enzymes, and other molecules.
Materials and Reagents
Appropriate growth media [e.g., MOPS minimal media (Neidhardt et al., 1974)]
50 mM NaHPO4, pH 6.7
Phosphate-buffered saline (PBS)
Talon Superflow metal affinity resin (Takara, catalog number: 635670; for purification of soluble proteins)
A stock solution of ~1 mM fluorescein 5' maleimide (FM; AnaSpec, catalog number: AS-81405) in anhydrous dimethyl formamide (DMF) or dimethyl sulfoxide (DMSO)
10 mM Tris-Cl, pH 8
β-mercaptoethanol (Fisher, catalog number: O3446I-100)
Sensor storage: solutions containing 10% glycerol, either labeled bacterial cells in media, or purified protein buffer, respectively. Store samples frozen at -70 °C.
Iron-deficient MOPS Minimal Medium (Neidhardt et al., 1974) (see Recipes)
Iron-free glassware (see Recipes)
50 mM sodium phosphate, pH 6.7 (see Recipes)
Phosphate-buffered saline (see Recipes)
Equipment
High-speed centrifuge (Beckman Coulter, model: Avanti J25)
Spectrophotometer (Beckman Coulter, model: DU800)
Fluorometer (OLIS Clarity; model: OLIS-SLM-Aminco 8100) and quartz fluorescence cuvettes (Fisher 14-958-128)
Typhoon Biomolecular Imager (GE/Amersham, model: 8600)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Newton, S. M. and Klebba, P. E. (2022). Fluorescent Binding Protein Sensors for Detection and Quantification of Biochemicals, Metabolites, and Natural Products. Bio-protocol 12(22): e4543. DOI: 10.21769/BioProtoc.4543.
- Kumar, A., Yang, T., Chakravorty, S., Majumdar, A., Nairn, B. L., Six, D. A., Marcondes Dos Santos, N., Price, S. L., Lawrenz, M. B., Actis, L. A., et al. (2022). Fluorescent sensors of siderophores produced by bacterial pathogens. J Biol Chem 298(3): 101651.
分类
微生物学 > 病原体检测 > 生物传感器
分子生物学 > 蛋白质 > 活性
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













