- EN - English
- CN - 中文
LIST: A Newly Developed Laser-assisted Cell Bioprinting Technology
LIST:新开发的激光辅助细胞生物打印技术
(*contributed equally to this work) 发布: 2022年10月05日第12卷第19期 DOI: 10.21769/BioProtoc.4527 浏览次数: 1930
评审: Alessandro DidonnaAnonymous reviewer(s)
Abstract
Cell bioprinting technologies aim to fabricate tissue-like constructs by delivering biomaterials layer-by-layer. Bioprinted constructs can reduce the use of animals in drug development and hold promise for addressing the shortage of organs for transplants. We recently introduced a laser-assisted drop-on-demand bioprinting technology termed Laser Induced Side Transfer (LIST). This technology can print delicate cell types, including primary neurons. This bioprinting protocol includes the following key steps: cell harvesting, bio-ink preparation, laser setup priming, printing, and post-printing analysis. This protocol includes a detailed description of the laser setup, which is a rather unusual setup for a biology lab. This should allow easy reproduction by readers with basic knowledge of optics. Although we have focused on neuron bioprinting, interested readers will be able to adapt the protocol to bioprint virtually any cell type.
Graphical abstract:

Background
Bioprinting technologies employ precise delivery of bio-inks for the fabrication of living constructs. Such constructs serve as drug screening models and can potentially address the organ donation shortage (Knowlton et al., 2018). Bioprinting technologies can be categorized into four main classes: material jetting, vat photopolymerization (e.g., stereolithography), pneumatic or mechanical material extrusion, and free-form spatial printing. Depending on the printing mechanism, these technologies present partial compatibility with available bio-ink formulations, with the bio-ink viscosity being the limiting factor (Kang et al., 2016).
Laser Induced Side Transfer (LIST) is a drop-on-demand bioprinting technology that was recently developed by our group (Ebrahimi Orimi et al., 2020; Roversi et al., 2021). This technology uses low-energy nanosecond laser pulses to generate a transient microbubble at the distal end of a glass microcapillary supplied with bio-ink. Microbubble expansion results in the ejection of a cell-laden microjet perpendicular to the irradiation axis. We have previously used LIST to print delicate cell types such as human umbilical vein endothelial cells (Ebrahimi Orimi et al., 2020) and adult mouse dorsal root ganglion (DRG) neurons (Roversi et al., 2021). Bioprinted cells maintained high viability and functionality. Compared to the first report on LIST (Ebrahimi Orimi et al., 2020), this protocol provides a detailed technical description of all steps, including the assembling of the laser setup, which is a rather unusual setup for a biology lab. This should allow easy reproduction by readers with basic knowledge of optics.
The LIST technology can be used to print virtually any cell type. As such, it holds promise to fill a technological gap in the drop-on-demand bioprinting field: the lack of technologies for printing large scale constructs using bio-inks of both high and low viscosity.
Materials and Reagents
0.45 μm filter (VWR, catalog number: CA28145-497)
15 mL Falcon tube (VWR, catalog number:62406-200)
100 μm filter (pluriSelect, catalog number: 43-40100-00)
Hollow square capillaries, ID 0.30 mm × 0.30 mm, 0.15 mm wall thickness, 50 mm long (Vitrocom, catalog number: 8330-050)
Tube for connecting the capillary to the pump (VWR, catalog number: 89404-042)
12-wells plate (VWR, catalog number: 10062-894)
18 mm microscope round cover glasses (VWR, catalog number: 48380-046)
35 mm high glass bottom dish (ibidi, catalog number: 81158)
60 mm Petri dishes (VWR, catalog number: 25384-092)
Homozygote lox-stop-lox-GCaMP6f (GCaMP6ffl/fl;) mice (Jackson Lab, catalog number: 028865)
TRPV1cre mice (Jackson Lab, catalog number: 017769)
Potassium chloride (KCl) (Sigma, catalog number: P3911), store in room temperature
Dulbecco's modified eagle medium (DMEM) (Thermo Scientific, Gibco, catalog number: 11965118), store at 4 °C
Fetal bovine (FB) essence (VWR/Avantor Seradigm, catalog number: 10803-034), store at -20 °C
Penicillin–streptomycin (Corning, catalog number: 30-002-CI), store at -20 °C
Collagenase A (Sigma, catalog number: 1108879300), store at -20 °C
Dispase® II (neutral protease, grade II) (Sigma, catalog number: 4942078001), store at 4 °C
Bovine serum albumin (BSA) culture grade (Fisher Scientific/Hyclone, catalog number: SH30574.02), store at 4 °C
Phosphate buffered saline (PBS) (Thermo Scientific, Gibco, catalog number: 10010023), store at room temperature
Neurobasal medium (Thermo Scientific, Gibco, catalog number: 21103049), store at 4 °C
XenoFree B-27TM supplement (Thermo Scientific, Gibco, catalog number: A1486701), store at -20 °C
Nerve growth factor (NGF) 2.5S subunit (Thermo Scientific, Gibco, catalog number: 13257019), store at -20 °C
Glial cell line–derived neurotrophic factor (GDNF) (Peprotech, catalog number: 450-51-10), store at -20 °C
Cytosine-beta-D-arabinofuranose hydrochloride (AraC) (Alfa Aesar, catalog number: J55671), store at -20 °C
Glass Pasteur pipette (Fisher Scientific, catalog number: 13-678-20B)
DNase (Sigma, catalog number: DN25), store at -20 °C
L-glutamine (VWR, catalog number: 02-0131), store at -20 °C
Basal medium (Millipore, catalog number: SCME001), store at -20 °C
Fibrinogen (Sigma-Aldrich, catalog number: F8630-5G), store at -20 °C
Allura red AC (Sigma-Aldrich, catalog number: 458848-100G), store at room temperature
Standard extracellular solution (Boston BioProducts, catalog number: C-3030F-4L), store at 4 °C
Capsaicin (Tocris, catalog number: 0462), store at room temperature
Thrombin (Sigma-Aldrich, catalog number: T7513-100UN), store at -20 °C
Supplemented DMEM (see Recipes), store at 4 °C
Collagenase/dispase II (see Recipes), store at -20 °C
BSA 15% (see Recipes), store at -20 °C
Supplemented neurobasal (see Recipes), store at 4 °C
Supplemented neurobasal with growth factors and AraC (see Recipes)
Equipment
Protective laser goggles for 532 nm (e.g., Thorlabs, catalog number: LG3)
Laser viewing card (Thorlabs, catalog number: VRC2)
Laser (Litron Lasers, Nano S 60-30, 532 nm)
Concave lens, f = -50 mm (Thorlabs, catalog number: LC1715-A-ML)
Convex lens, f = 100 mm (Thorlabs, catalog number: LA1509-A)
Half-wave plate (Thorlabs, catalog number: WPMH05M-633). For optimal performance, use a half-wave plate tailored for 532 nm (e.g., Thorlabs, catalog number: WPH05M-532)
Rotating stage (Thorlabs, catalog number: PRM1Z8)
DC servo motor controller (Thorlabs, catalog number: TDC001)
Polarizing beam splitter (e.g., Thorlabs, catalog number: PBS25-532)
Broadband dielectric mirrors (Thorlabs, catalog number: BB1-E02)
Mechanical shutter (Thorlabs, catalog number: SH05)
K-cube solenoid controller (Thorlabs, catalog number: KSC101)
Beam splitter, 10:90 (R:T) (Thorlabs, catalog number: BSN10)
Si detector (photodiode) (Thorlabs, catalog number: DET10A)
Pyroelectric sensor (Gentec-eo, catalog number: QE12LP-S-MB)
Concave lens, f = −50 mm (Thorlabs, catalog number: LC1715-A-ML)
Convex lens, f = 150 mm (Thorlabs, catalog number: LA1433-A-ML)
UVFS beam splitter, 70:30 (R:T) (Thorlabs, catalog number: BST10R)
4× objective lens, plan achromat-NA = 0.1 (Olympus, catalog number: RMS4X)
XYZ motorized translational stage (Thorlabs, catalog numbers: PT1-Z8+MAX 201)
Two-channel APTTM stepper motor controller (Thorlabs, catalog number: BSC102)
K-cube brushed DC servo motor controller (Thorlabs, catalog number: KDC101)
Syringe pump (New Era Pump Systems Inc., catalog number: NE-1000)
High speed camera (Kron Technologies, Chronos 1.4)
LED illumination (Thorlabs, catalog number: MCWHL5)
T-cube LED driver (Thorlabs, catalog number: LEDD1B)
UVFS beam splitter, 50:50 (R:T) (Thorlabs, catalog number: BSW10R)
3D printed capillary holder (see the CAD file as Supplementary material 1) and 6.8 mm × 1.9 mm securing O ring.
Colored glass filter, 570 nm longpass (Thorlabs, catalog number: FGL570)
Achromatic lens, f = 150 mm (Thorlabs, catalog number: AC254-150-A-ML)
Kinematic fluorescence filter cube, left turning (Thorlabs, catalog number: DFM1L)
Kinematic fluorescence filter cube, light turning (Thorlabs, catalog number: DFM1)
Kinematic mirror mount for Ø1" optics (Thorlabs, catalog number: KM100)
Lens mount with retaining ring for Ø1" optics (Thorlabs, catalog number: LMR1)
Incubator, air jacketed CO2 incubator (VWR, catalog number: 10810-888)
Centrifuge, 5810R (Eppendorf, catalog number: 022628089)
Water bath (PolyScience, catalog number: WBE20A11B)
Hemocytometer (VWR, catalog number: 76299-416)
Stereomicroscope (Nikon, catalog number: SMZ-745T)
P1000 pipette (VWR, catalog number: 89079-974)
Tweezers and dissection tools (World Precision Instruments, catalog number: 504167)
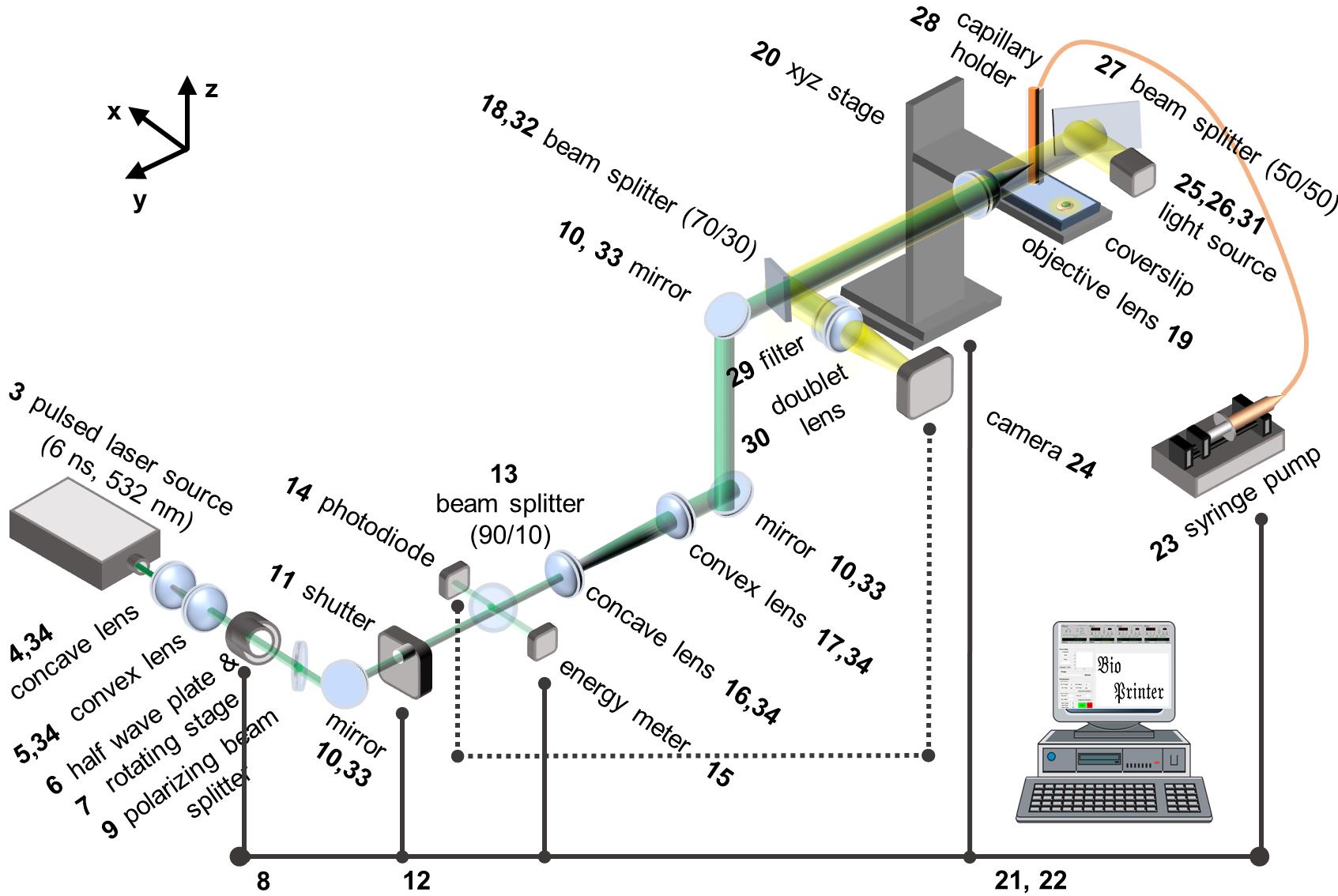
Figure 1. Schematic of laser-induced side transfer (LIST). The numbering of the items corresponds to that of the Equipment section.
Software
MATLAB 2020a (MathWorks, https://www.mathworks.com/)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Roversi, K., Ebrahimi Orimi, H., Erfanian, M., Talbot, S. and Boutopoulos, C. (2022). LIST: A Newly Developed Laser-assisted Cell Bioprinting Technology. Bio-protocol 12(19): e4527. DOI: 10.21769/BioProtoc.4527.
分类
生物工程 > 生物打印
生物工程 > 生物医学工程 > 药物递送
生物科学 > 生物技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link