- EN - English
- CN - 中文
Collection of Xylem Exudates from the Model Plant Arabidopsis and the Crop Plant Soybean
从模式植物拟南芥和农作物大豆中收集木质部分泌物
发布: 2022年10月05日第12卷第19期 DOI: 10.21769/BioProtoc.4520 浏览次数: 2730
评审: Ashish RanjanPooja VermaMalgorzata LichockaAnonymous reviewer(s)

相关实验方案
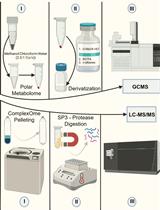
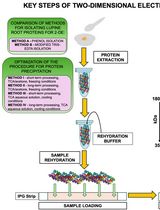
利用SP3珠和稳定同位素质谱技术优化蛋白质合成速率:植物核糖体的案例研究
Dione Gentry-Torfer [...] Federico Martinez-Seidel
2024年05月05日 2843 阅读
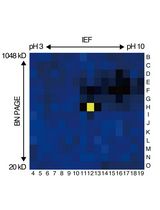
基于活性蛋白质组学和二维聚丙烯酰胺凝胶电泳(2D-PAGE)鉴定拟南芥细胞间隙液中的靶蛋白酶
Sayaka Matsui and Yoshikatsu Matsubayashi
2025年03月05日 1949 阅读
Abstract
A number of molecules, such as secreted peptides, have been shown to mediate root-to-shoot signaling in response to various conditions. The xylem is a pathway for water and molecules that are translocated from roots to shoots. Therefore, collecting and analyzing xylem exudates is an efficient approach to study root-to-shoot long-distance signaling. Here, we describe a step-by-step protocol for the collection of xylem exudate from the model plant Arabidopsis and the crop plant soybean (Glycine max). In this protocol, we can collect xylem exudate from plants cultured under normal growth conditions without using special equipment.
Graphical abstract:

Xylem exudates on the cut surfaces of an Arabidopsis hypocotyl and a soybean internode.
Background
Plants consist of multiple organs that play distinctive roles during plant life. Hence, organ-to-organ communication is indispensable for plants to adapt to environmental stresses and maintain homeostasis at the whole-plant level. It is known that various types of macromolecules, such as phytohormones, RNAs, proteins, and peptides mediate long-distance signaling (Okamoto et al., 2015, 2016; Lu et al., 2020). The xylem plays an important role in the translocation of water and micronutrients from roots to shoots. In addition, long-distance signaling molecules, including small secreted peptides and phytohormones, are translocated from roots to shoots through the xylem. Therefore, collecting and analyzing xylem exudates is an effective approach to identify novel long-distance mobile molecules.
To date, several methods have been developed to collect xylem exudates (Buhtz, 2004, Goodger et al., 2005; Dafoe and Constabel, 2009), and the appropriateness of a given method is dependent on plant species and conditions. One method uses a pressure chamber to obtain xylem exudate from woody plants. In this method, roots are placed inside a sealed chamber, and pressurized gas is added to the chamber. This method can be applied to woody plants that have mechanically stronger tissues than herbaceous plants, and a precise protocol was written by Flajšman et al. (2017). Another method depends on root pressure and is usually applied to herbaceous plants whose tissues are soft. Root pressure is one of the driving forces of xylem sap. Accumulation of solutes in root xylem results in low water potential and generates positive hydrostatic pressure in the xylem (Taiz et al., 2015). Although the quantity of xylem exudates obtained with this method depends on plant species and conditions, this method requires no special equipment and has been applied to many herbaceous plants, including Arabidopsis thaliana (Iwai et al., 2003; Buhtz et al., 2004; Kehr et al., 2005; Alvarez et al., 2006; Djordjevic et al., 2007; Ligat et al., 2011; Ko et al., 2014; Tabata et al., 2014; Okamoto et al., 2015; Luo and Zhang, 2019). However, the collection of xylem exudates is likely not familiar to many researchers, and it is difficult to find a step-by-step protocol for this method.
Previously, we collected xylem exudate from soybean to conduct peptidome analysis and identified various endogenous root-to-shoot mobile peptides (Okamoto et al., 2015). In addition, we analyzed xylem exudate from the model plant Arabidopsis thaliana and detected mature CLAVATA3/ESR-related 2 peptides (Okamoto et al., 2022a). In this protocol, we describe step-by-step procedures for the collection of xylem exudates from both the model plant Arabidopsis and the crop plant soybean.
Part I. Collection of xylem exudates from Arabidopsis
Materials and Reagents
1.5 mL tube (BIO-BIK, catalog number: ST-0150F)
Plastic square dish (140 mm × 100 mm, height 18 mm/EIKEN, catalog number: AW2000)
Plastic Petri dish (ø 85 mm, height 20 mm/EIKEN, catalog number: AP2310)
Razor blade (FEATHER, FA-10)
Micropipette [Nichiryo, catalog number: 00-NPX2-2 (0.1–2 µL)]
Arabidopsis seeds
Ethanol (FUJIFILM Wako, catalog number: 057-00451)
Tween 20 (MP Biomedicals, catalog number: 194841)
Sodium hypochlorite solution (FUJIFILM Wako, catalog number: 197-02206)
Murashige and Skoog (MS) plant salt mixture (FUJIFILM Wako, catalog number: 392-00591)
Gamborg’s vitamin solution 1,000× (Sigma-Aldrich, catalog number: G1019)
Sucrose (FUJIFILM Wako, catalog number: 196-00015)
Agar (FUJIFILM Wako, catalog number: 016-11875)
Culture media (100 mL) (see Recipes)
Equipment
Growth chamber (Nippon Medical & Chemical Instruments, model: LH-241S)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
OKamoto, S. and Kawasaki, A. (2022). Collection of Xylem Exudates from the Model Plant Arabidopsis and the Crop Plant Soybean. Bio-protocol 12(19): e4520. DOI: 10.21769/BioProtoc.4520.
分类
植物科学 > 植物生物化学 > 蛋白质 > 分离和纯化
生物化学 > 蛋白质 > 分离和纯化
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link