- EN - English
- CN - 中文
A Simplified Paradigm of Late Gestation Transient Prenatal Hypoxia to Investigate Functional and Structural Outcomes from a Developmental Hypoxic Insult
妊娠晚期短暂性产前缺氧的简化范式研究发育性低氧损伤的功能和结构结果
发布: 2022年10月05日第12卷第19期 DOI: 10.21769/BioProtoc.4519 浏览次数: 2065
评审: Oneil Girish BhalalaHélène LégerAnonymous reviewer(s)

相关实验方案
基于 rAAV-α-Syn 与 α-Syn 预成纤维共同构建的帕金森病一体化小鼠模型
Santhosh Kumar Subramanya [...] Poonam Thakur
2025年12月05日 1583 阅读
Abstract
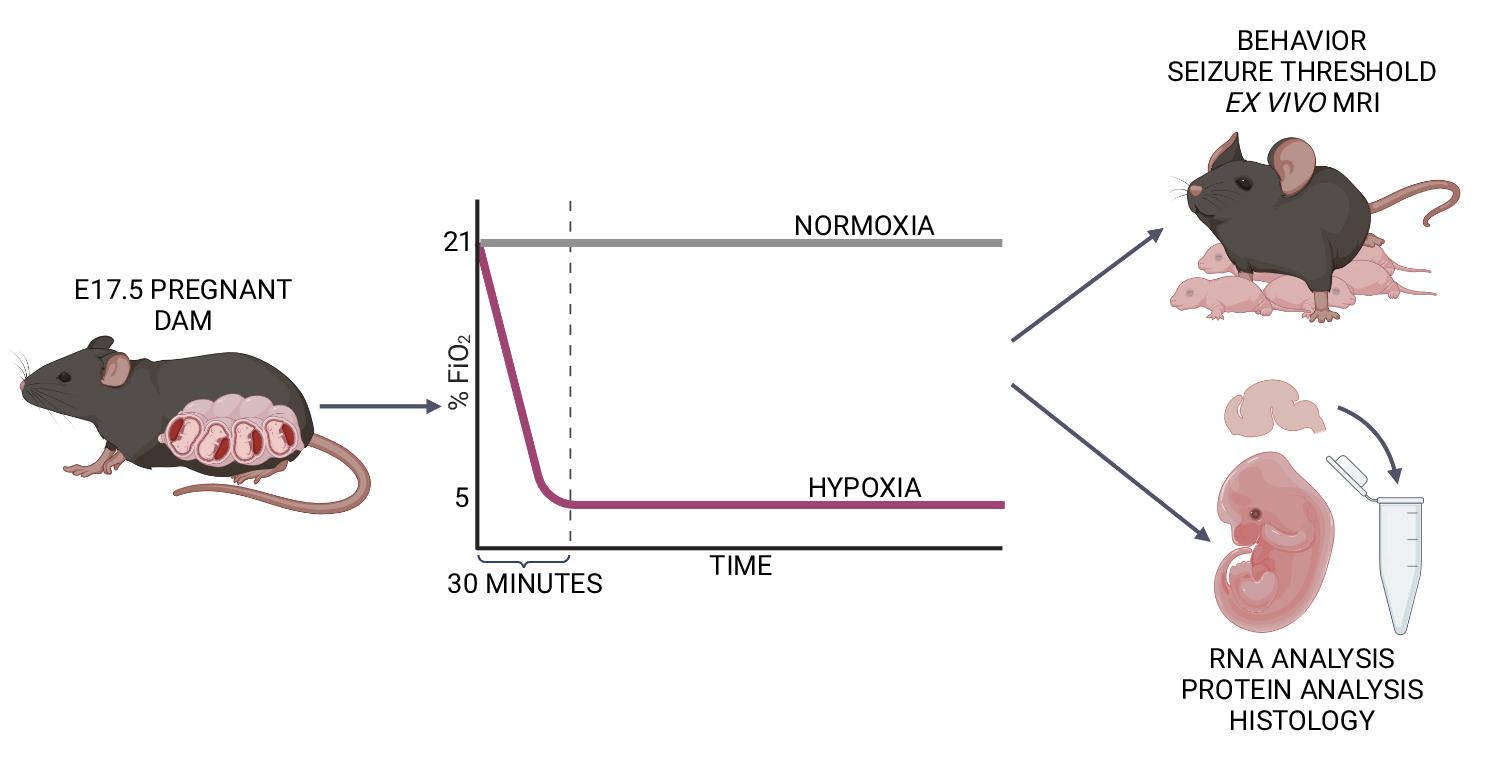
Late-gestation transient intrauterine hypoxia is a common cause of birth injury. It can lead to long-term neurodevelopmental disabilities even in the absence of gross anatomic injury. Currently, postnatal models of hypoxia–ischemia are most commonly used to study the effect of oxygen deprivation in the fetal brain. These models, however, are unable to take into account placental factors that influence the response to hypoxia, exhibit levels of cell death not seen in many human patients, and are unable to model preterm hypoxia. To address this gap in research, we have developed a protocol to induce transient hypoxia in fetal mice. A pregnant dam at gestational day 17.5 is placed into a hypoxia chamber. Over 30 min, the inspired oxygen is titrated from 21% (ambient air) to 5%. The dam remains in the chamber for up to 8 h, after which fetal brains can be collected or pups delivered for postnatal studies. This protocol recapitulates phenotypes seen in human patients exposed to transient in utero hypoxia and is readily reproducible by researchers.
Graphical abstract:
Background
Each year, millions of neonates are affected by intrauterine hypoxia, of which the most recognized form is hypoxic ischemic encephalopathy (HIE) in full-term infants (Lee et al., 2013; Stanaway et al., 2018). Prenatal hypoxia is one of the leading worldwide causes of long-term brain injury (Lee et al., 2013; Stanaway et al., 2018), and HIE is characterized by an intrapartum loss of oxygen and nutrients. Approximately 40% of affected children develop a neurodevelopmental disability (NDD), such as autism or epilepsy (Ferriero, 2004; Lee et al., 2013; Stanaway et al., 2018). Therapeutic hypothermia is the mainstay of current therapy but is only available to a fraction of children who have moderate to severe acute HIE and are near a facility capable of initiating it in the immediate perinatal period (Ahearne et al., 2016). Children initially classified as having mild HIE may still experience adverse outcomes (de Haan et al., 2006; van Kooij et al., 2010; Eunson, 2015; Reiss et al., 2019; Schreglmann et al., 2020; Finder et al., 2020). These mildly affected children do not qualify for therapeutic hypothermia. In addition to term infant HIE, HIE in preterm infants is a poorly understood entity that predisposes children to NDDs (Schmidt and Walsh, 2010; Gopagondanahalli et al., 2016). Preterm infants with HIE are also not eligible for therapeutic hypothermia. Given the need for targeted therapies, we must develop animal models that can recapitulate the full spectrum of phenotypes exhibited by children affected by prenatal hypoxia.
Existing models of transient hypoxia in rodents have focused primarily on its effect on the postnatal brain (postnatal day 7–10) (Rice et al., 1981; Sun et al., 2016) because this postnatal period is considered to correlate neuroanatomically to term human infants (Semple et al., 2013). Models such as the Vannucci model involve unilateral ligation of the carotid artery with subsequent exposure to hypoxia (Rice et al., 1981; Sun et al., 2016). In addition to recapitulating human neuroanatomy, this postnatal method makes studying the combined effects of hypoxia and ischemia more technically feasible.
While there are benefits to postnatal rodent models of transient hypoxia, these have limitations. Importantly, postnatal models do not account for maternal and placental factors that may modulate the fetal response to hypoxia. The in utero environment is hypoxic at baseline (Trollmann et al., 2008), and many studies investigating the hypoxic response have revealed complex interactions between oxygen control and injury outcome (Tomita et al., 2003; Chen et al., 2008; Sheldon et al., 2009; Sheldon et al., 2014; Arthuis et al., 2017; Xu et al., 2019; Peebles et al., 2020; Zhang et al., 2021). Additionally, postnatal models involving carotid artery ligation followed by hypoxia result in severe injury, characterized by significant levels of cell death (Rice et al., 1981), and not the milder injury seen in many human patients (Lee et al., 2013). Finally, while the postnatal rodent brain correlates anatomically with term human infants, some functional networks mature faster in postnatal rodents than in term infants (Feather-Schussler and Ferguson, 2016). At birth, mice exhibit neuroanatomy analogous to infants born at 23–26 weeks gestation (Semple et al., 2013). However, they do not display respiratory or feeding issues common in children born before 34 weeks gestation (Zhao et al., 2019). Additionally, mice are ambulatory around postnatal day 10, a milestone human children do not achieve until approximately 12 months old (Feather-Schussler and Ferguson, 2016; Aziz et al., 2018).
Although previous mouse models of prenatal hypoxia have been described (Baud et al., 2004; Mallard and Vexler, 2015), many are primarily used to study the effects of chronic hypoxia throughout gestation. Other prenatal models of transient injury are more challenging to execute (such as late gestation uterine artery ligation) (Kubo et al., 2017). Sheep models of transient and mild hypoxia-only injury require specialized equipment, ample resources, and are not amenable to genetic manipulation (McClendon et al., 2017; McClendon et al., 2019). Here, we characterize a protocol to induce transient, mild, hypoxic injury in prenatal mice.
Previously, we have used this model to characterize the effect of late gestation (embryonic day 17.5) transient prenatal hypoxia (5% FiO2) on long-term neurodevelopmental and anatomical features in mice (Cristancho et al., 2022). Our studies showed increased levels of hypoxia-inducible factor 1 alpha (a marker of hypoxic exposure) in the brains of fetal mice exposed to prenatal hypoxia. Mice exposed to hypoxia had decreased weights at postnatal day 2. Hypoxic exposure also led to decreased seizure threshold in mice. In addition, both male and female hypoxic mice showed abnormalities in grip strength and repetitive behaviors. Finally, male hypoxic mice had increased anxiety-like behaviors, while female hypoxic mice showed abnormalities in social interaction. We hope that future researchers may use this model to investigate the mechanisms underlying mild and preterm HIE and elucidate the maternal–placental–fetal factors underlying neurodevelopmental outcomes in preterm children exposed to transient hypoxia.
Materials and Reagents
Medical NF grade nitrogen, size 200 cylinder, CGA-580 (Airgas, NI NF200). Protect from light and store in a well-ventilated space; indefinite shelf life.
Soda lime, indicating (Thermo Fisher Scientific, catalog number: AA4478636). Store at room temperature; stored indefinitely in an airtight container (at least 3 years). Use fresh soda lime for each exposure.
Hydrogel (clear H2O, 70-01-5022). Store at room temperature; once opened, store in a sealed plastic bag and discard after one week or when dried out.
Laboratory rodent diet 5015 pellets (Lab Diet, catalog number: 001328). Store at room temperature in climate-controlled conditions; shelf life is at least nine months.
Nalgene VERSI-DRY lab table soakers (VWR, catalog number: 52857-104). Store at room temperature; indefinite shelf life.
VWR disposable Petri dishes, 10 cm, semi-stackable (VWR, catalog number: 25384-088). Store at room temperature; indefinite shelf life.
Alcohol 70% (ethyl alcohol), 4 L (Millipore Sigma, catalog number: 65350-85). Store at room temperature in flammables cabinet; will last multiple years if kept sealed away from light.
C57BL/6NCrl mice at embryonic day 17.5 were used for this protocol (Charles River)
We have also successfully used C57BL6/J (Jackson Laboratories) animals.
We have not tested other gestational time points of exposure but believe the protocol could be adapted depending on the experimental needs.
Equipment
ProOx 360 versatile high infusion rate O2 controller with oxygen sensor (Biospherix, model: E702)
Harris model 425-200-580 heavy duty argon, helium, and nitrogen single stage regulator, CGA-580 (Airgas, model: HCL3000773)
Animal cage enclosures with riser platform (Biospherix, A-Chamber)
Translucent plastic bins (to house animals during hypoxic exposures) [e.g., BINO plastic storage bins, deep large, The Handler Collection (Amazon, 12093-CLR)]
Cutting board or similar divider (to visually separate animals when performing two hypoxic exposures in one chamber) [e.g., Dexas superboard pastry board, 14 in. × 17 in. (Amazon, B000063SRL)]
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Gadra, E. C. and Cristancho, A. G. (2022). A Simplified Paradigm of Late Gestation Transient Prenatal Hypoxia to Investigate Functional and Structural Outcomes from a Developmental Hypoxic Insult. Bio-protocol 12(19): e4519. DOI: 10.21769/BioProtoc.4519.
分类
神经科学 > 神经系统疾病 > 动物模型
神经科学 > 神经系统疾病 > 细胞机制
细胞生物学 > 组织分析 > 损伤模型
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link