- EN - English
- CN - 中文
Split-Chloramphenicol Acetyl Transferase Assay to Study Protein-Protein Interactions and Ubiquitylation in Escherichia coli
分裂氯霉素乙酰转移酶测定法研究大肠杆菌中的蛋白质-蛋白质相互作用和泛素化
(*contributed equally to this work) 发布: 2022年09月05日第12卷第17期 DOI: 10.21769/BioProtoc.4497 浏览次数: 3403
评审: David PaulNeha NandwaniAnonymous reviewer(s)

相关实验方案

基于蛋白筛选策略从合成文库中分离抗原特异性纳米抗体:结合 MACS 的酵母展示筛选与 FLI-TRAP 方法
Apisitt Thaiprayoon [...] Dujduan Waraho-Zhmayev
2026年01月20日 473 阅读
Abstract
Protein-protein interactions and protein modifications play central roles in all living organisms. Of the more than 200 types of post-translational modifications, ubiquitylation is the most abundant, and it profoundly regulates the functionality of the eukaryotic proteome. Various in vitro and in vivo methodologies to study protein interactions and modifications have been developed, each presenting distinctive benefits and caveats. Here, we present a comprehensive protocol for applying a split-Chloramphenicol Acetyl-Transferase (split-CAT) based system, to study protein-protein interactions and ubiquitylation in E. coli. Functional assembly of bait and prey proteins tethered to the split-CAT fragments result in antibiotic resistance and growth on selective media. We demonstrate assays for protein interactions, protein ubiquitylation, and the system response to small compound modulators. To facilitate data collection, we provide an updated Scanner Acquisition Manager Program for Laboratory Experiments (SAMPLE; https://github.com/PragLab/SAMPLE) that can be employed to monitor the growth of various microorganisms, including E. coli and S. cerevisiae. The advantage posed by this system lies in its sensitivity to a wide range of chloramphenicol concentrations, which allows the detection of a large spectrum of protein-protein interactions, without the need for their purification. The tight linkage between binding or ubiquitylation and growth enables the estimation of apparent relative affinity, and represents the system’s quantitative characteristics.
Graphical abstract:
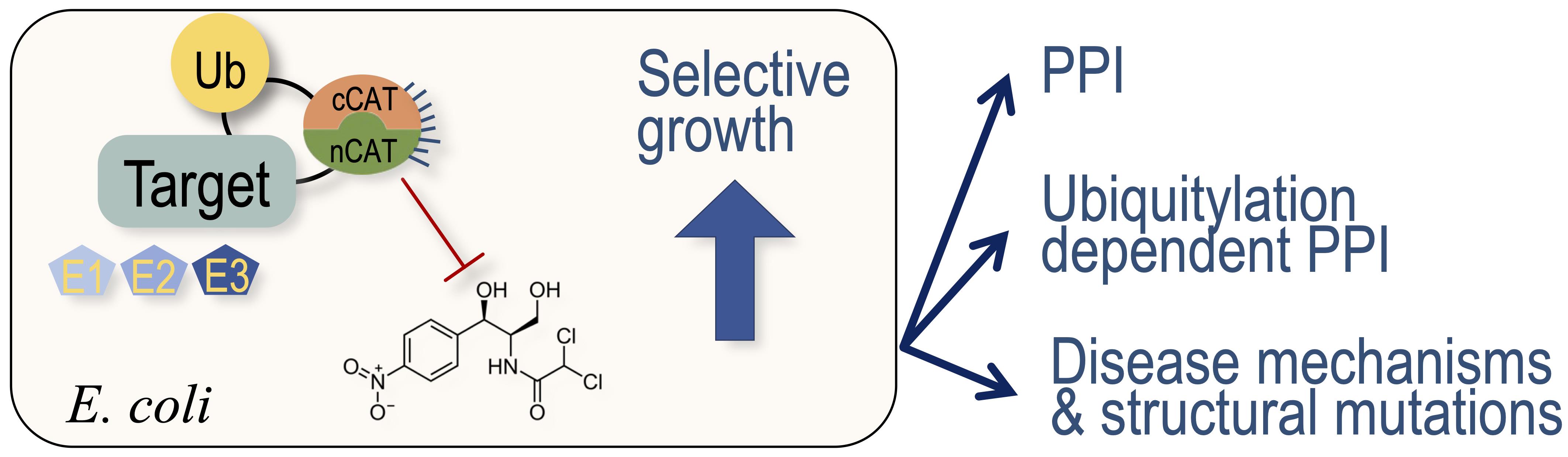
Background
Most cellular processes are carried out and controlled by protein-protein interactions (PPIs) and post-translation protein modifications (PTMs). Therefore, detecting PPIs and PPI-dependent PTMs is critical for understanding normal and pathological conditions, and for potential therapeutic development. Indeed, many methodologies have been developed for identification and characterization of PPIs and PTMs. Each of these methods has its own benefits and caveats. In the 1980s, phage display and genetic yeast two-hybrid systems were developed to study PPIs (Smith, 1985; Fields and Song, 1989; Bair et al., 2008). These methods allowed, for the first time, a high-throughput screening of a large repertoire of proteins or peptides. A great benefit of these methods is the linkage to the DNA sequence encoding the identified polypeptide. However, in the phage display systems, proteins interact in the extra-cellular environment, a process that leads to many false positive interactions, due to misfolding. In contrast, complementation of the split-Gal4 transcription factor in the yeast nucleus is required to activate the reporter, and consequently limits the positive readouts of the screen. Cleverly, Stagljar and co-workers circumvented this with a split-ubiquitin system [a system previously developed by Johnsson and Varshavsky (Johnsson et al., 1994)], in which the interaction of membrane proteins induces a proteolytic cleavage that releases a fused nuclear transcription factor, activating reporter genes in the nucleus (Stagljar et al., 1998). Additionally, Michnick and co-workers developed two excellent Protein-fragment Complementation Assays (PCA) based on split dihydrofolate reductase (DHFR) and split-TEM-1 (β-lactamase) enzymes (Pelletier et al., 1998; Galarneau et al., 2002). The split-DHFR system provides growth phenotypic readouts, both in bacteria and yeast. However, seeding of fairly low cell concentrations is required to prevent non-specific growth (Levin-Kravets et al., 2021). Split-reporters that provide fluorescence or luminescence are widely used to detect PPIs. Forster resonance energy transfer by fluorescence lifetime imaging (FRET-FLIM) and bimolecular fluorescence complementation (BiFC) are useful in live cell imaging (Majoul et al., 2002; Walter et al., 2004). The advantage of this approach is the information regarding the cellular localization of the PPIs. Nevertheless, these methods require sophisticated microscopy equipment, which can frequently be a limiting factor.
Eukaryotic protein interactions are often regulated by transient PTMs that challenge the identification of these PPIs. The high sensitivity of mass-spectrometry technologies are beneficial for the detection of a large spectra of PPIs and PTMs (Blagoev et al., 2003; Peng et al., 2003). However, redundancy within the modifier enzymes challenges their linking to their respective targets. Protein microarray serves as an alternative, comprehensive approach for identification of PTMs, while linking them to their modifier enzyme (Gupta et al., 2007). Sequence and structure-based in silico approaches provide predictions of PPIs, and thus facilitate analysis using the above described methods (Marcotte et al., 1999; London et al., 2012; Keren-Kaplan et al., 2013; Jumper et al., 2021). Among over 200 different PTMs in the eukaryotic proteome, ubiquitylation is the most abundant and complex modification, with over 600 ubiquitin E3-ligases in humans. Here, we present a detailed protocol for a recently developed application to detect and quantify PPIs and ubiquitylation, using a recombinant system that is expressed in E. coli, and based on Split-chloramphenicol Acetyl-Transferase (Split-CAT) (Levin-Kravets et al., 2021). Bacterial growth efficiency is the system readout, which can be estimated as endpoint, or continuously measured to obtain kinetics readouts. Contradictory to selection systems that report growth based on synthesis of essential metabolites, such as uracil, thymidine, or histidine, which pose limits on seeding concentration, the Split-CAT system modifies the antibiotic, thus allowing high-density seeding of bacteria. The sensitivity to a wide concentration range of chloramphenicol allows the detection of a large spectrum of affinities of PPIs. The tight correlation between binding or ubiquitylation and growth efficiency allows the estimation of apparent relative affinity (Keren-Kaplan et al., 2016). The system was constructed with several compatible plasmids, which provides modularity, and facilitates its transfer to different bacteria (Figure 1).
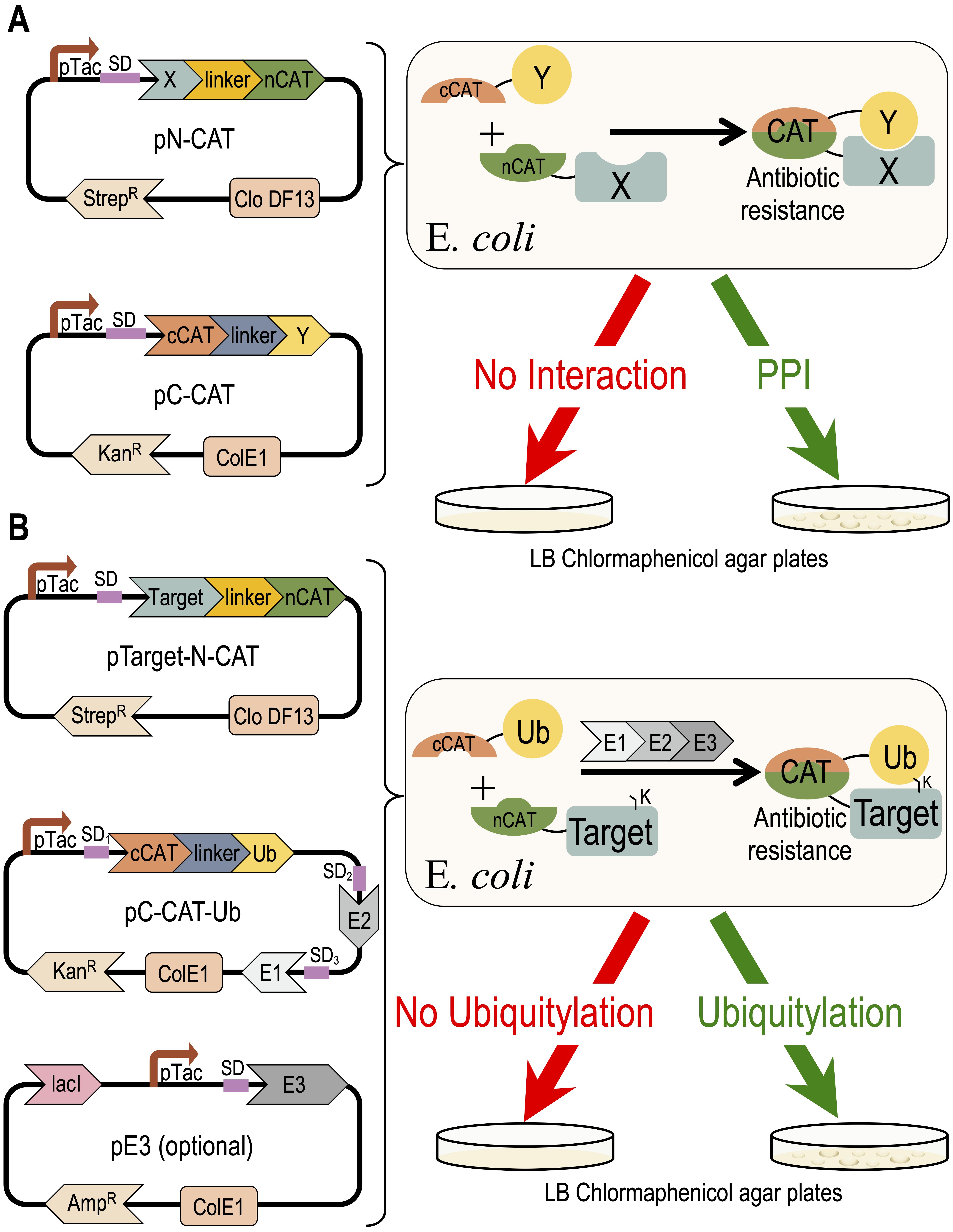
Figure 1. Concept of the Split-CAT selection system. The DNA plasmids encoding the system are shown on the left side. The activities of the proteins encoded by the system are shown to the right. (A) Shows a scheme of the PPI study. (B) Shows a scheme of the ubiquitylation study.
Split-CAT as reporter in self-ubiquitylation assay
Here, we demonstrate how to harness the Split-CAT system for a self-ubiquitylation assay, using the ubiquitin E3-ligase NleG6-2 of EHEC as an example. In this assay, we co-express the E3-ligase along with a cognate ubiquitylation cascade, including ubiquitin, E1, and E2 in a 96-well plate, or in an agar Petri dish (Figure 2). In this system, the NleG6-2 serves both as a ligase and as a ubiquitylation target, and therefore it was fused to the N-CAT fragment. The C-CAT fragment is fused to ubiquitin. Upon self-ubiquitylation, a stable covalent isopeptide bond between the C-terminus of ubiquitin and a lysine residue in the E3-ligase facilitates functional assembly of the split-CAT, giving rise to CAM resistance, and growth on selective media (Figure 2A). As a negative control, we co-express the system without the E1 and E2 enzymes (∆E1, ∆E2 in Figure 2B). In this scenario, an attenuated E. coli growth is seen, due to antibiotic tolerance only in low antibiotic concentrations. Without CAM (curve with light blue circles), the growth of the strain with self-ubiquitylation (resistance) is identical to the ∆E1, ∆E2 strain (tolerance). But what is the optimal CAM concentration to run the assay? We found that expression of different protein cascades provides different optimal CAM concentrations. Here, we describe how to identify the optimal CAM concentration for further experiments, where one wishes to study the effect of mutations, small molecule modulators, and more on the ubiquitylation process. We recommend setting the experiment up with increased CAM concentrations, varied from zero to approximately 32 μg/mL (as shown in Figure 2). Cumulative growth is the integral (area under curve) of the growth curve in each of the CAM concentrations (Figure 2C and 2F). This provides a single value that represents the growth efficiency. A plot of the growth efficiencies against CAM concentrations (similar to an IC50 curve) is shown in Figure 2C. The growth efficiency of the strain with ‘resistance’ (light blue) is more significant than that of the strain with ‘tolerance’ (red). Subtraction of the two curves (Res.–Tol.) results in an optimum curve (Figure 2D). The maximum of the curve in Figure 2D provides an optimal CAM concentration for downstream experiments, where one can study the effect of mutants or small molecule modulators.
Materials and Reagents
Tubes 1.5, 15, 50 mL (LIFEGENE catalog numbers: LMCT1.7B, LTB15, LTB50)
Multichannel pipet (Gilson PIPETMAN L Multichannel P12x200L, catalog number: FA10012)
LB agar (1.5% agar, BD DifcoTM Agar, catalog number: 11793523)
Petri dishes 90, 50 mm (MINIPLAST, catalog numbers: 820-090-01-017, 872-050-05-000)
Black matte spray paint for plastic Petri dish covers (RUST-OLEUM 2X Ultra Cover Ultra Matte Spray)
96-well plate (CORNING, catalog number: 3596)
Highly efficient E. coli competent cells, recA- strain (such as Mach1 or DH5α; ThermoFisher, catalog number: C862003 and 18265017 respectively)
Gibson assembly mix (NEB, catalog number: E2611S)
DNA plasmids: pC-CAT-Ub, pTarget-N-CAT and pE3 (available from our laboratory for academic use).
Antibiotics such as ampicillin, kanamycin, streptomycin, and chloramphenicol (FORMEDIUM, catalog number: AMP25, CAISSON LABS, catalog number: 05212005, CHEM-IMPEX INTERNATIONAL, catalog number: 0028, FORMEDIUM, catalog number: CLA01)
Luria-Bertani (LB) medium (NEOGEN, catalog number: NCM0173A)
SOC medium (FORMEDIUM, catalog number: SOC0201)
Equipment
Spectrophotometer (MRC, model: Spectro-V11D)
Vortex (Scientific Industries, model: Vortex-Genie 2, catalog number: SI-0236)
Thermostatic water bath (Fried Electric, WBS)
Incubators (for Petri dishes and shaker for tubes, MRC LABORATORY-INSTRUMUNTS, catalog number: BOD-80, INFORS HT, Ecotro1)
Flatbed office (US-letter/A4) scanner (such as Epson, model: Perfection V19 or V37)
Plate reader-shaker-incubator (TECAN, Sunrise)
Software
SAMPLE (https://github.com/PragLab/SAMPLE)
Fiji/ImageJ (https://fiji.sc or https://imagej.nih.gov/ij/) and its plugin Time Series Analyzer V3 (Balaji J. 2007); https://imagej.nih.gov/ij/plugins/time-series.html)
Analysis software such as Excel, KaleidaGraph, GraphPad Prism or SigmaPlot
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Florentin, A., Kordonsky, A., Yariv, E., Avishid, R., Efron, N., Akogwu, E. and Prag, G. (2022). Split-Chloramphenicol Acetyl Transferase Assay to Study Protein-Protein Interactions and Ubiquitylation in Escherichia coli. Bio-protocol 12(17): e4497. DOI: 10.21769/BioProtoc.4497.
分类
生物化学 > 蛋白质 > 相互作用
微生物学 > 微生物生物化学 > 蛋白质
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










