- EN - English
- CN - 中文
Maximizing the Rod Outer Segment Yield in Retinas Extracted from Cattle Eyes
最大化从牛眼中提取的视网膜中的杆外段产量
发布: 2022年07月20日第12卷第14期 DOI: 10.21769/BioProtoc.4474 浏览次数: 2135
评审: Gal HaimovichSrinidhi Rao Sripathy RaoAnonymous reviewer(s)
Abstract
The retina is a thin neuronal multilayer responsible for the detection of visual information. The first step in visual transduction occurs in the photoreceptor outer segment. The studies on photoreception and visual biochemistry have often utilized rod outer segments (OS) or OS disks purified from mammalian eyes. Literature reports several OS and disk purification procedures that rarely specify the procedure utilized to collect the retina from the eye. Some reports suggest the use of scissors, while others do not mention the issue as they declare to utilize frozen retinas. Because the OS are deeply embedded in the retinal pigmented epithelium (RPE), the detachment of the retina by a harsh pull-out can cause the fracture of the photoreceptor cilium. Here, we present a protocol maximizing OS yield. Eye semi-cups, obtained by hemisecting the eyeball and discarding the anterior chamber structures and the vitreous, are filled with Mammalian Ringer. After 10–15 min of incubation, the retinas spontaneously detach with their wealth of OS almost intact. The impressive ability of the present protocol to minimize the number of OS stuck inside the RPE, and therefore lost, compared with the classic procedure, is shown by confocal laser scanning microscopy analysis of samples stained ex vivo with a dye (MitoTracker deep red) that stains both retinal mitochondria and OS. Total protein assay of OS disks purified by either procedure also shows a 300% total protein yield improvement. The advantage of the protocol presented is its higher yield of photoreceptor OS for subsequent purification procedures, while maintaining the physiological features of the retina.
Keywords: Bovine (牛)Background
The vertebrate eye is a photoreceptive sense organ (Stenkamp, 2015). Its laminar structure comprises three main layers: the fibrous tunic, consisting of the cornea and sclera; the vascular tunic (uvea), including the iris, ciliary body, and choroid; and the nervous tunic, including the retina and the retinal pigmented epithelium (RPE) (Netter, 2018). The eye can also be divided into an anterior and a posterior segment. The former includes the cornea, iris, ciliary body, and lens, while the latter encompasses the vitreous, retina, RPE, and choroid. The RPE faces the sensory retina and separates the subretinal space from the choroid, forming the outer blood–retinal barrier. Aqueous humor fills the anterior segment. The mammalian retina, a part of the central nervous system, consists of five classes of neurons (retinal ganglion cells, amacrine cells, bipolar cells, horizontal cells, and the cone and rod photoreceptors), arranged into three nuclear and two synaptic layers (Masland, 2012). The input for visual signaling (Palczewski, 2014) comes from photoreceptor cells, distinct in cones, responsible for the chromatic vision, and rods, high-sensitive receptors responsible for scotopic vision, that consist of a specialized inner and outer segment. Most mammalian retinas express one short wavelength-sensitive and one long wavelength type of cone, while a few express three (Masland, 2012). A cilium connects the OS to the inner segment (IS) (Gilliam et al., 2012); in fact, rod and cone OS are structurally homologous to non-motile cilia. The OS captures photons, initiating the visual transduction (Palczewski, 2014). In particular, the rod OS consists of a stack of approximately 2,000 membranous disks surrounded by a plasma membrane, which express the proteins devoted to phototransduction (Molday and Moritz, 2015). Recently, it was reported that the proteins of the mitochondrial redox chain are also functionally expressed in the rod OS (Bruschi et al., 2020). Disks are synthesized at the base of the OS (Young, 1967) and are shed at the apical tip, where the RPE phagocytizes exhausted disks (Campbell et al., 2018). Since the 1970s, studies on photoreception and on the biochemistry of vision have typically utilized rod outer segments (OS) or OS disks from cattle eyes, as these allow to obtain a large quantity of sample. Literature reports several rod OS purification procedures (de Grip et al., 1972; Raubach et al., 1974; McDowell and Kühn, 1977; Papermaster, 1982; Zimmerman and Godchaux, 1982; Molday et al., 1987; Uhl et al., 1987; Bubis, 1998), and one isolation procedure for disks (Smith et al., 1975, 1982). However, these seldom specify the procedure utilized to collect the retina from the eye. Some reports suggest the use of scissors; others do not include this passage, as they utilize frozen retinas. Because the OS are deeply embedded in the RPE, the detachment of the retina from the RPE by a harsh procedure can cause the fracture of the photoreceptor cilium. The goal of this protocol is to describe a gentle procedure for the extraction of living retinas from bovine eyeballs, maximizing the yield of rod OS, and avoiding the fracture of the rod at the cilium level inside the RPE and their eventual loss when the eye semi-cup is discarded.
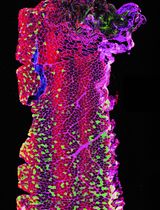
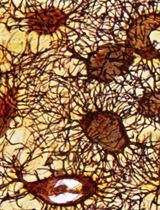
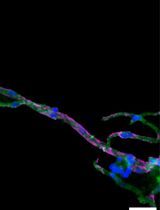
The best yield of rod OS or disks is obtained when retina is let to spontaneously detach from the RPE by filling the eye semi-cup with sterile filtered mammalian ringer (MR) for at least 15 min. In the first step of the protocol, cattle eyes, freed from periocular muscle and connective tissue, are cut at the level of the ora serrata on a section plane below the pupil, as shown in Figure 1, and the anterior portion of the eye is discarded. In this way, it is possible to eliminate the lens, the aqueous humor, and the vitreous humor, obtaining a semi-cup containing the retina (Figure 1). Then, to facilitate the spontaneous detachment of the retina, the semi-cup is filled with sterile filtered MR and incubated for at least 10 min: in this way, most of the rod OS remain attached to the free-floating retina that detaches from the RPE. By contrast, if the retina is extracted utilizing a tweezer or a soft brush, the photoreceptors can fracture at the cilium and remain immersed in the RPE. The comparison between the two methods is shown by data obtained from the confocal laser scanning microscope (CLSM) analysis shown in Figures 3 and 4, after staining the retina with MitoTracker Deep Red 633 (ThermoFisher), as previously reported (Calzia et al., 2010; Panfoli et al., 2010; Ravera et al., 2007). Specifically, the retina detached by the MR incubation method presents a homogeneous layer of photoreceptors (Figure 3, Panel A) while, in the remaining semi-cup of the eye, only mitochondria are visible (Figure 3, Panel B). By contrast, when the retina is detached with a soft brush, few recognizable OS are observed (Figure 4, Panel A), which are instead still partially stained inside the eye semi-cup (Figure 4, Panel B).

Figure 1. Scheme for the cut of the eye semi-cup. The drawing shows where the plane of section passes to obtain the eye semi-cup. The panel on the right shows a close-up of an eye semi-cup unscrewed by the crystalline lens, aqueous humor, and vitreous humor, in which the retina lining the bottom of the eye is recognized.
The innovation of the present protocol, with respect to the literature, is the use of a gentle procedure to extract the retinas from the eye semi-cup, maximizing the absolute amount of rod OS still attached to the retina. Confocal laser scanning microscopy (Figures 3 and 4) demonstrated the advantage our protocol for the extraction of the retinas offers, with respect to the conventional extraction of the retina by means of a tweezer or even using a soft brush, and the actual risk of leaving almost all the rod OS inside the RPE of the eye semi-cup (Figure 3).
The significance of the improvement represented by the present protocol is the possibility to maximize the presence of the photoreceptor OS in the retinas extracted from large mammalian eye semi-cups. This is advantageous if retinas are used to purify rod OS or disks, but also for any subsequent procedure requiring the presence of photoreceptors in the isolated retina.
Materials and Reagents
Eppendorf Tube (Eppendorf, catalog number: 0030 102.002)
Glass Pasteur Pipettes (Teklab Limited, UK catalog number: GP225)
Centrifuge tube, conical bottom, 13 mL volume (VWR, catalog number: VWRI525-0179)
Milli-Q® Biocel System water (Millipore, http://www.millipore.com/)
Ampicillin (Millipore, catalog number: 171257), storage at -20°C
Protease inhibitor cocktail (Millipore, catalog number: 539133), storage at -20°C
NaCl (VWR, catalog number: 7647-14-5), room temperature storage
KCl (VWR, catalog number: 7447-40-7), room temperature storage
Na2HPO4 (Millipore, catalog number: 567547), room temperature storage
NaH2PO4 (Millipore, catalog number: 567545), room temperature storage
MgCl2·6H2O (Millipore, catalog number: 442615), room temperature storage
CaCl2·2H2O (Millipore, catalog number: 137101), room temperature storage
Steritop® Bottle-top Filtration Units 0.22 μm (Millipore Express® PLUS)
MitoTrackerTM Deep Red 633 FM Dye (ThermoFischer Scientific, catalog number: M46753)
Mammalian Ringer (MR) (see Recipes)
MitoTrackerTM Deep Red 633 FM Dye stock solution (see Recipes)
Equipment
Pipetting Standard (Gilson, Pipetman®, models: P20, P200, and P1000) (http://www.gilson.com/Products/)
MicroCentrifuge (Eppendorf, model: 5417R)
Dissecting Scalpels and Blades, dissecting Scissors, dissecting dressing Forceps, as in United (ScientificTM Dissecting Set, catalog number: S111010)
Dewar flask
Red light/dark room
Vacuum filtration device
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Panfoli, I., Calzia, D., Ravera, S., Bianchini, P. and Diaspro, A. (2022). Maximizing the Rod Outer Segment Yield in Retinas Extracted from Cattle Eyes. Bio-protocol 12(14): e4474. DOI: 10.21769/BioProtoc.4474.
分类
神经科学 > 感觉和运动系统 > 视网膜
细胞生物学 > 组织分析 > 组织分离
细胞生物学 > 组织分析 > 组织成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link