- EN - English
- CN - 中文
Binding Affinity Quantifications of the Bacteriophage Mu DNA Modification Protein Mom Using Microscale Thermophoresis (MST)
使用微尺度热泳 (MST) 对噬菌体 Mu DNA 修饰蛋白Mom的结合亲和力定量
发布: 2022年07月20日第12卷第14期 DOI: 10.21769/BioProtoc.4472 浏览次数: 3678
评审: ASWAD KHADILKARMarc-Antoine SaniAnonymous reviewer(s)
Abstract
Epigenetic modifications play diverse roles in biological systems. Nucleic acid modifications control gene expression, protein synthesis, and sensitivity to nucleic acid-cleaving enzymes. However, the mechanisms underlying the biosynthesis of nucleic acid modifications can be challenging to identify. Studying protein-ligand interactions helps decipher biosynthetic and regulatory pathways underlying biological reactions. Here, we describe a fluorescence labeling-based quantitative method for unraveling the biomolecular interactions of bacteriophage Mu DNA modification protein Mom with its ligands, using microscale thermophoresis (MST). Compared to traditional methods for studying protein-biomolecular interactions, MST requires significantly lower sample amounts, volumes, and analysis time, thus allowing screening of a large number of candidates for interaction with a protein of interest. Another distinguishing feature of the method is that it obviates the need for protein purification, often a time- and resource-consuming step, and works well with whole or partially purified cell extracts. Importantly, the method is sensitive over a broad range of molecular affinities while offering great specificity and can be used to interrogate ligands ranging from metal ions to macromolecules. Although we established this method for a DNA modification protein, it can easily be adapted to study a variety of molecular interactions engaged by proteins.
Keywords: Microscale thermophoresis (MST) (微尺度热泳(MST))Background
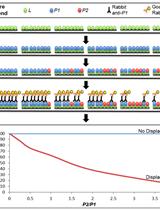
[Background] Biological background: Biological entities have evolved a plethora of epigenetic modifications in their nucleic acids, ranging from simple methylation to complex moieties derived from sugars, amino acids, polyamines, and other cellular metabolites (Boccaletto et al., 2018; Sood et al., 2019). Epigenetic modifications regulate gene expression, mRNA translation, and cellular stress responses, and protect bacteriophages from DNA-damaging immune systems of bacterial hosts (Kahmann, 1983; Hattman, 1999; Choi et al., 2014; Gu et al., 2014; Reynolds et al., 2014; Bryson et al., 2015; Hutinet et al., 2019). The ability to target nucleic acid modifications has broad applications in designing antibiotics and antifungal strategies through the impairment of modification pathways unique and essential to pathogens (Koh and Sarin, 2018). Similarly, the fields of phage therapy and microbiome engineering stand to benefit from the discovery of DNA modification pathways that can protect the DNA payload from a wide range of host immune systems. Novel DNA modifications are constantly being discovered in phages, presenting new puzzles surrounding their biosynthesis and function (Lee and Weigele, 2021; Lee et al., 2022). Studying molecular interactions opens a window into the inner workings of macromolecules, cells, and underlying mechanistic and regulatory pathways (Corbeski et al., 2018; Karambelkar et al., 2020). Here, we describe interaction studies of the DNA modification protein Mom in the context of its metal ion and small molecule cofactor interactions (Karambelkar et al., 2020) using the technique of microscale thermophoresis (MST).
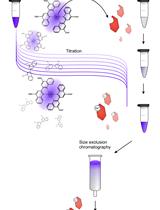
Technical background: MST offers a simple method to quantitatively investigate protein-ligand interactions, and is based on thermophoresis, the movement of molecules across thermal gradients (Jerabek-Willemsen et al., 2011). Ligand-induced changes in size, charge, hydration shell, or conformation of a macromolecule alter its thermophoretic movement (Jerabek-Willemsen et al., 2011). Using a titration approach, MST enables the measurement of affinity constants of a wide variety of interactions in the binding equilibrium (Karambelkar et al., 2020; Osuna et al., 2020a, 2020b). MST requires extremely small sample amounts compared to conventional methods like isothermal calorimetry (ITC) or surface plasmon resonance (SPR), obviates ligand immobilization, and works over a broad range of binding affinities.
Another notable strength of MST over conventional approaches is its compatibility with complex mixtures and cell extracts (Lippok et al., 2012; Khavrutskii et al., 2013; Bartoschik et al., 2018). The ability to fluorescently tag a specific his-tagged protein of interest in a complex mixture of proteins, as highlighted in this protocol, not only obviates protein purification, often a rate limiting and cumbersome step, but also provides high sensitivity for detecting interactions in a near-native environment (Bartoschik et al., 2018).
Although MST measurements can be performed using intrinsic fluorescence of proteins (Seidel et al., 2012), labeling of the target proteins with a suitable fluorophore is required when using complex samples (Bartoschik et al., 2018). In routine labeling techniques for MST, fluorophores are covalently attached to lysine residues using NHS- or to cysteine residues using maleimide chemistry. However, these labeling methods require purified proteins and cannot be applied to a mixture of proteins. Moreover, it is not possible to predict where the fluorophore will bind to the protein—inhomogeneous protein-dye conjugates might even display destabilization, loss of functionality, or steric hindrance at the ligand binding site (Lindhoud et al., 2012). The above limitations are overcome by the selective and site-specific labeling of a protein of interest in a complex mixture. This can be achieved in various ways, such as by expressing in vivo a fusion of the protein of interest with a fluorescent protein like GFP (Khavrutskii et al., 2013; Magnez et al., 2017; Gao et al., 2021). However, such relatively large tags are not always desired for quantitative interaction analysis and also require the cloning of a suitable linker and fluorescence tag. Various other site-specific labeling strategies have been demonstrated, such as co-translational introduction of unnatural or modified amino acids, or labeling via specific amino acid sequences, including His-tags and tetracysteine motifs (Griffin et al., 1998; Krishnan et al., 2007; Nienberg et al., 2016). Among these, the His-tag is the most convenient, popular, and widely used affinity tag for purification, immobilization, or detection of proteins.
The tris-NTA/His-tag system comprises one of the smallest high-affinity recognition elements known to date (Braner et al., 2016). Fast, stoichiometric binding of tris-NTA conjugates enables in situ protein labeling of His-tagged proteins, making the system suitable for quantitative protein interaction analysis by MST (Lata et al., 2005). Because the tris-NTA/His-tag labeling is based on non-covalent coordinate bonds with the transition metal ion Ni(II), it is sensitive to reagents that compete or interfere with the labeling (Table 1).
Materials and Reagents
Protein Purification
15 mL Bioruptor TPX tubes (Diagenode, catalog number: NC1463349)
10 kDa dialysis membrane (Sigma-Aldrich, catalog number: 0530-100FT)
Isopropyl-β-D-thiogalactoside (Sigma-Aldrich, catalog number: 11411446001)
Lysozyme (Sigma-Aldrich, catalog number: L6876)
Phenylmethanesulfonyl fluoride (PMSF) (Sigma-Aldrich, catalog number: P7626)
Poly(ethyleneimine) (Sigma-Aldrich, catalog number: P3143)
NaCl (Sigma-Aldrich, catalog number: 746398)
Sodium phosphate dibasic (Sigma-Aldrich, catalog number: S5136)
Sodium phosphate monobasic (Sigma-Aldrich, catalog number: S3139)
EDTA (Sigma-Aldrich, catalog number: E5134)
2-Mercaptoethanol (Sigma-Aldrich, catalog number: M3148)
HEPES (Affymetrix USB, catalog number:16928)
Ammonium sulfate (Sigma-Aldrich, catalog number: A4418)
Cellulose phosphate (Sigma-Aldrich, catalog number: C3145)
Trizma base or Tris base (Sigma-Aldrich, catalog number: T6066)
NaOH (SDFCL, catalog number: 20252KO5)
HCl (Qualigens, catalog number: Q29147)
Amicon Ultra-15 centricon10 kDa NMWCO (Millipore, catalog number: UFC901008)
Bovine serum albumin (BSA), analytical standard (Millipore Sigma, catalog number: P5619)
Lysis buffer (see Recipes)
Phosphocellulose binding buffer (see Recipes)
Wash buffer 1 (see Recipes)
Wash buffer 2 (see Recipes)
Elution buffer (see Recipes)
Storage buffer (see Recipes)
MST Experiment
PCR plates (Axygen, catalog number: 14-222-320)
Ammonium Iron(II) sulfate (Sigma-Aldrich, catalog number: 09719)
Acetyl Coenzyme A (Sigma-Aldrich, catalog number: A2056)
MonolithTM NT.115 Series, Premium Capillaries (NanoTemper Technologies GmbH, catalog number: MO-K025–200 Count)
Monolith His-Tag Labeling Kit RED-Tris-NTA (NanoTemper Technologies GmbH, catalog number: MO-L008)
MST buffer (see Recipes)
1× PBS-T (see Recipes)
Equipment
Incubator (New Brunswick Scientific Innova 4230)
Bioruptor® Sonicator (Diagenode UCD 300)
Centrifuge (Kubota 6500)
Ultracentrifuge (Beckman L8-70M)
Magnetic Stirrer (IKA Big Squid)
Peristaltic pump (Pharmacia LKB pump P-1)
Pipettes (GilsonTM PIPETMAN ClassicTM Pipets P2, 20, 200, and 1000) (Gilson, catalog numbers: F144801, F123600G, F123601G, and F123602G)
Monolith NT.115 instrument (NanoTemper Technologies GmbH)
Software
MO.Control 2 from Nanotemper Technologies (https://nanotempertech.com/monolith-mo-control-software/)
MO.Affinity Analysis 3 from Nanotemper Technologies (https://nanotempertech.com/monolith/monolith-nt115/)
GraphPad Prism 5 version 5.03 (https://www.graphpad.com/scientific-software/prism/)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Udupa, S., Nagaraja, V. and Karambelkar, S. (2022). Binding Affinity Quantifications of the Bacteriophage Mu DNA Modification Protein Mom Using Microscale Thermophoresis (MST). Bio-protocol 12(14): e4472. DOI: 10.21769/BioProtoc.4472.
分类
微生物学 > 微生物生物化学
生物化学 > 蛋白质 > 相互作用 > 蛋白质-配体相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link