- EN - English
- CN - 中文
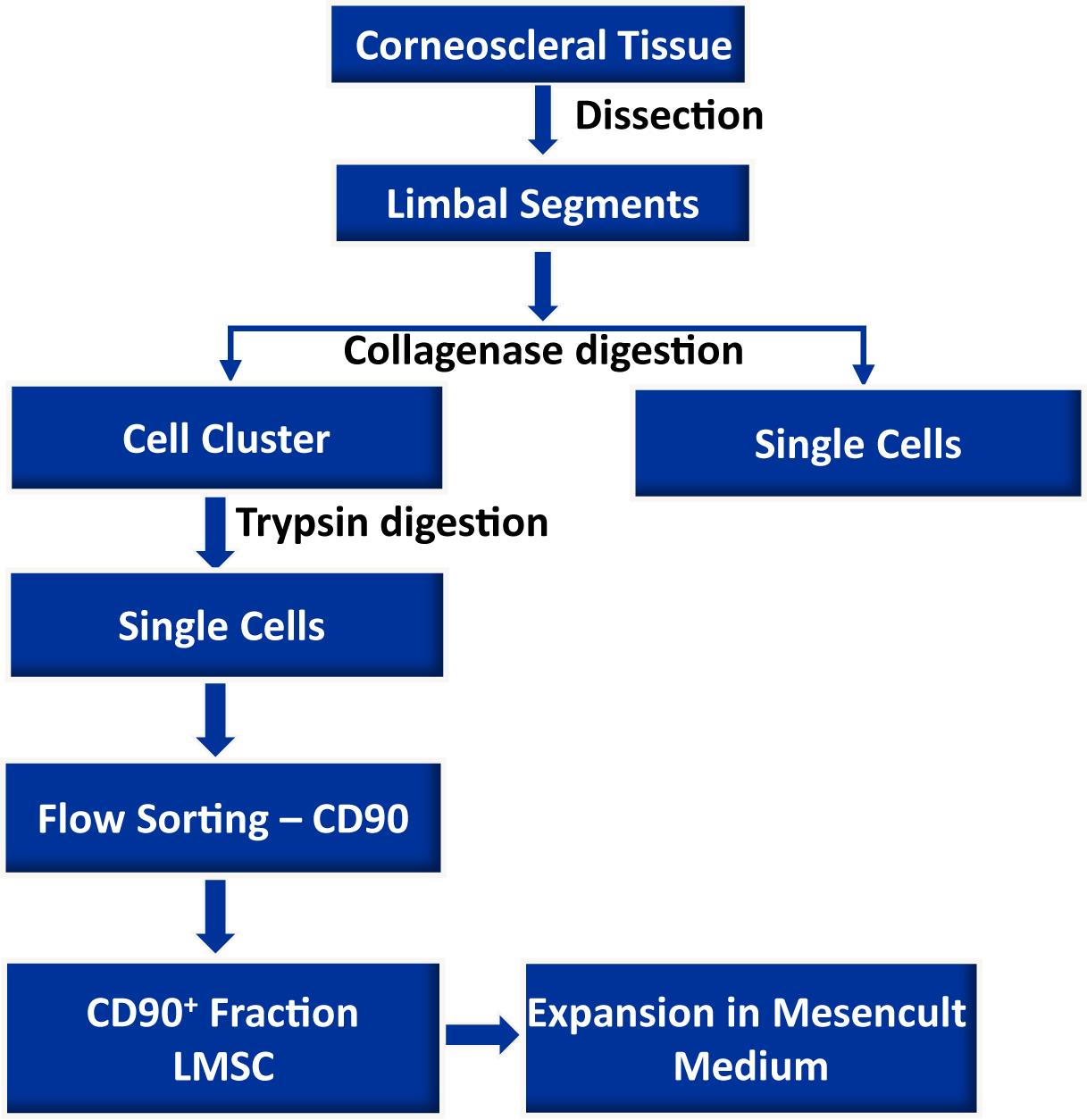
Isolation and ex vivo Expansion of Limbal Mesenchymal Stromal Cells
边缘间充质基质细胞的分离和离体扩增
发布: 2022年07月20日第12卷第14期 DOI: 10.21769/BioProtoc.4471 浏览次数: 2968
评审: Vivien Jane Coulson-ThomasMingxia SunIsabel Moreno

相关实验方案

来自骨髓增生性肿瘤患者的造血祖细胞的血小板生成素不依赖性巨核细胞分化
Chloe A. L. Thompson-Peach [...] Daniel Thomas
2023年01月20日 2363 阅读
Abstract
Limbal mesenchymal stromal cells (LMSC), a cellular component of the limbal stem cell niche, have the capability of determining the fate of limbal epithelial progenitor cells (LEPC), which are responsible for the homeostasis of corneal epithelium. However, the isolation of these LMSC has proven to be difficult due to the small fraction of LMSC in the total limbal population, and primary cultures are always hampered by contamination with other cell types. We recently published the efficient isolation and functional characterization of LMSC from the human corneal limbus using CD90 as a selective marker. We observed that flow sorting yielded a pure population of LMSC with superior self-renewal capacity and transdifferentiation potential, and supported the maintenance of the LEPC phenotype. Here, we describe an optimized protocol for the isolation of LMSC from cadaveric corneal limbal tissue by combined collagenase digestion and flow sorting with expansion of LMSC on plastic.
Graphical abstract:
Background
Homeostasis of the corneal epithelium is regulated by limbal epithelial stem/progenitor cells (LEPC) located at a specific anatomic location referred to as the limbal stem cell niche (Gonzalez et al., 2018). It is characterized by limbal vasculature, a specific extracellular matrix composition (ECM), and surrounding non-epithelial limbal niche cells (LNCs) (Shortt et al., 2007; Ordonez et al., 2012; Polisetti et al., 2016) (Figure 1). Limbal mesenchymal stromal cells (LMSC), a cellular component of the limbal stem cell niche, have been shown to support corneal epithelial regeneration during wound healing, and the maintenance of the LEPC phenotype, both in vitro and in vivo (Dziasko et al., 2014; Nakatsu et al., 2014; Li et al., 2018; Polisetti et al., 2016). In addition, LMSC were shown to have potent immunomodulatory, anti-inflammatory, and anti-angiogenic properties, making them potentially attractive tools for clinical use (Funderburgh et al., 2016; Veréb et al., 2016; Al-Jaibaji et al., 2019; Polisetti et al., 2021). Thus, the co-cultivation of LEPC with LMSC might represent an improved strategy to generate cell transplants for patients suffering from limbal stem cell deficiency (Rama et al., 2017; Ghareeb et al., 2020). Previously, LMSC have been isolated either by enzymatic digestion of limbal tissue (dispase, collagenase, or in combination) or by explant culture of limbal tissue followed by enrichment using cell type-specific media (Chen et al., 2011; Xie et al., 2012; Li et al.,2012; Li et al., 2014; Chen et al., 2015; González et al., 2013; Polisetty et al., 2008; Xiao et al., 2020). The main disadvantage of these methods is the contamination by other cell types (Polisetty et al., 2008; Li et al., 2012). Collagenase digestion of limbal tissue results in cell clusters consisting of 80% epithelial cells and 20% LMSC, whereas a combination of dispase and collagenase yields clusters composed of approximately 95% LMSC (Li et al., 2012). Thus, current protocols for LMSC purification require culturing of at least one passage to eliminate contaminating cells. Thus, research on freshly isolated LMSC has been hampered by the lack of a good protocol for isolating this cell type.
Here, we present an optimized protocol for the isolation of LMSC from organ-cultured corneal samples by means of fluorescence-activated cell sorting (FACS), using CD90 as a selective marker (Polisetti et al., 2022).
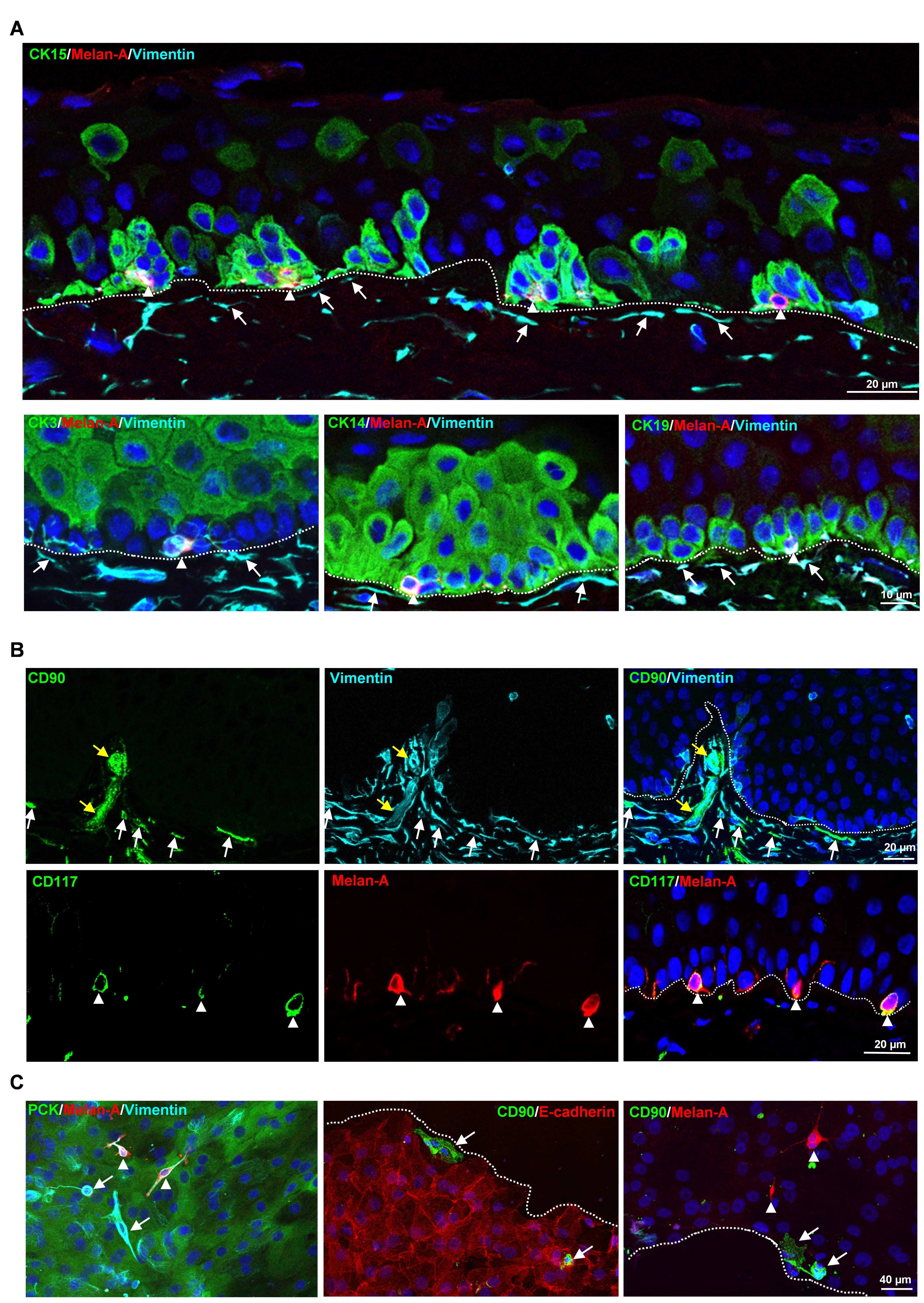
Figure 1. Localization of limbal niche cells in situ. A. Triple immunostaining analysis of limbal tissue sections showing melanocytes [melan-A+ (red) vimentin+ (cyan) cells, arrow heads] in close contact with clusters of cytokeratin (CK)15+, CK14+, CK19+ (green) limbal epithelial progenitor cells (LEPC), whereas sub-epithelial stromal cells [vimentin+ cells (cyan), arrows] were in close association with basal limbal epithelial cells, and not with more superficial CK3+ cells (green). Dashed line represents the basement membrane (BM), and nuclear counterstaining was done with 4′,6-diamidino-2-phenylindole (DAPI, blue). B. Double immunostaining of limbal sections showing the co-localization of CD90 (green) and vimentin (cyan) in the sub-epithelial stromal cells (white arrows), which were in close association with basal layers of limbal epithelium (dotted line represents the BM), as well as blood vessels of the limbal stroma (yellow arrows). The limbal sections also show the co-localization of CD117 (green) and melan A (red) in the melanocytes (arrow heads) at the basal layer of the limbal epithelium. Nuclear counterstaining with DAPI (blue). C. Immunofluorescence analysis of cultured limbal clusters showing the expression of keratins (PCK, green) and vimentin (cyan) in epithelial cells, melan-A (red) and vimentin (cyan) expression in melanocytes (arrow heads), and only vimentin expression in stromal cells (arrows). Double immunostaining of cultured limbal clusters showing the CD90+ stromal cells (green, arrow) at the edge of clusters and also in between E-cadherin+ epithelial cells (red, dashed line represents the edge of the cluster), whereas melan-A+ melanocytes were located between the cells (red, arrow heads). Nuclear counterstaining with DAPI (blue). Reprinted from Polisetti et al. (2022), licensed under a CC BY 4.0.
Materials and Reagents
12-well plate (Corning, Costar®, catalog number:3513)
Micropipette tips (0.5–20 µL, 100–200 µL, 1,000 µL) (Greiner Bio-One)
60 mm cell culture dish (Corning, Falcon®, catalog number: 353004)
100 mm cell culture dish (Corning, Falcon®, catalog number: 353003)
Syringe filter 0.2 μm (VWR, catalog number: 28145-501)
Disposable Scalpel blades No. 10 (pfm Medical ag, Feather®, catalog number: 201000010)
Serological pipettes (5 mL, 10 mL) (Corning, StripetteTM)
15 mL conical tubes (Greiner Bio-One, catalog number:188271)
50 mL conical tubes (Greiner Bio-One, catalog number:227261)
T75 flasks (Corning, catalog number: CLS430641)
Reversible cell strainers (Stem Cell Technologies, catalog number:27215)
Cell filter 20 µm (Cell TricsTM, Sysmex Partec GmbH, catalog number:04-004-2325)
FACS tubes (5 mL polystyrene round-bottom tube, Falcon, catalog number: 352058)
Collagenase A (Sigma-Aldrich, Roche Diagnostics, catalog number: 10103578001)
Dulbecco’s Phosphate Buffered Saline (DPBS) (no calcium, no magnesium) (Thermo Fisher Scientific, GibcoTM, catalog number: 14190094)
0.25% Trypsin-EDTA (Thermo Fisher Scientific, Gibco®, catalog number: 25200056)
Dulbecco’s Modified Eagle Medium (DMEM) high glucose (Thermo Fisher Scientific, GibcoTM, catalog number: 11960044)
Fetal Bovine Serum (FBS) (Thermo Scientific, GibcoTM, catalog number: 10082147)
Penicillin-Streptomycin (Sigma-Aldrich, catalog number: P4333)
MesenCultTM MSC Basal Medium (Human) (Stemcell Technologies, catalog number: 05401)
MesenCultTM MSC Stimulatory Supplement (Human) (Stemcell Technologies, catalog number: 05402)
70% Ethanol
0.5 M EDTA (Invitrogen, catalog number: AM9260G)
CD11c-PE (Biolegend, catalog number: 337205)
CD14-PE (Biolegend, catalog number: 301805)
CD19-PE (Biolegend, catalog number: 302207)
CD44-PE (Biolegend, catalog number: 397503)
CD45-PE (Biolegend, catalog number: 304007)
CD73-PE (Biolegend, catalog number: 344003)
CD90-PE (Abcam, catalog number: 328109)
CD90-APC (BD biosciences, catalog number: 559869)
CD105-PE (Biolegend, catalog number: 323205)
Mouse IgG2a, k Isotype-APC (eBioscience, catalog number: 17-4724-81)
Mouse IgG2a, k Isotype-PE (Biolegend, catalog number: 400212)
Cytokeratin AE1/AE3 (DAKO, catalog number:M3515)
Melan-A (Abcam, catalog number: EPR20380)
Vimentin (R&D Systems, catalog number: MAB2105)
4′,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich, catalog number: MBD0015)
Collagenase solution (see Recipes)
Mesencult complete medium (see Recipes)
FACS Buffer (see Recipes)
Equipment
Pipette aid (BRAND, accu-jet® pro)
Micropipette (Eppendorf Research plus, P20, P200, P1000)
Forceps (Dumont, 5-Dumoxel®-H)
Hemocytometer (MARIENFELD, Neubauer, catalog number: 0640130)
Biosafety cabinet (Thermo ScientificTM, Type S2020 1.2)
CO2 incubator (Thermo ScientificTM, HeracellTM 240i)
Centrifuge (Thermo ScientificTM, Heraeus Multifuge 1S-R)
Phase contrast inverted microscope with a camera (ZEISS, Objectives 4×, 10×, 20×)
Freezer -20°C (Liebherr)
Refrigerator 2–8°C (Siemens)
FACS Aria II sorter (BD Biosciences)
Water bath (GFL®, catalog number: 1013)
Software
CapturePro 2.10.0.1 (JENOPTIC Optical systems GmbH)
FACSDiva software (BD Pharmingen, BD Biosciences)
FlowJo software (Tree Star)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Polisetti, N., Sharaf, L., Reinhard, T. and Schlunck, G. (2022). Isolation and ex vivo Expansion of Limbal Mesenchymal Stromal Cells. Bio-protocol 12(14): e4471. DOI: 10.21769/BioProtoc.4471.
分类
干细胞 > 成体干细胞 > 间充质干细胞
生物工程 > 生物医学工程
细胞生物学 > 细胞分离和培养 > 细胞分离 > 流式细胞术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link











