- EN - English
- CN - 中文
A Robust Nanoparticle-based Magnetic Separation Method for Intact Lysosomes
一种用于分离完整溶酶体的基于纳米颗粒的磁分离方法
发布: 2022年07月05日第12卷第13期 DOI: 10.21769/BioProtoc.4453 浏览次数: 3261
评审: Alessandro DidonnaAmberley D. StephensAnonymous reviewer(s)
Abstract
Lysosome isolation is a preresiquite for identifying lysosomal protein composition by mass spectroscopic analysis, to reveal lysosome functions, and their involvement in some diseases. Magnetic nanoparticle-based fractionation has received great attention for lysosome isolation, owing to its high efficiency, purity, and preservation of lysosomal structures. Understanding the intracellular trafficking of magnetic probes is the key point of this technique, to determine the appropriate time for magnetic isolation of lysosomes, because this parameter changes depending on different cell lines used. The traditional magnetic probes, such as superparamagnetic iron oxide nanoparticles (SPIONs), require surface modification by fluorescent dyes to enable the investigation of their intracellular trafficking, which has some disadvantages, including the possible alternation of their bio-interaction, and the instability of fluorescence properties in the lysosomal environment. To overcome those limitations, we present a protocol that employs magnetic-plasmonic nanoparticles (MPNPs) to investigate intracellular trafficking using their intrinsic imaging capability, followed by quick lysosome isolation using a magnetic column. This protocol can be easily applied to isolate the intact lysosomes of any adherent cell lines.
Graphical abstract:
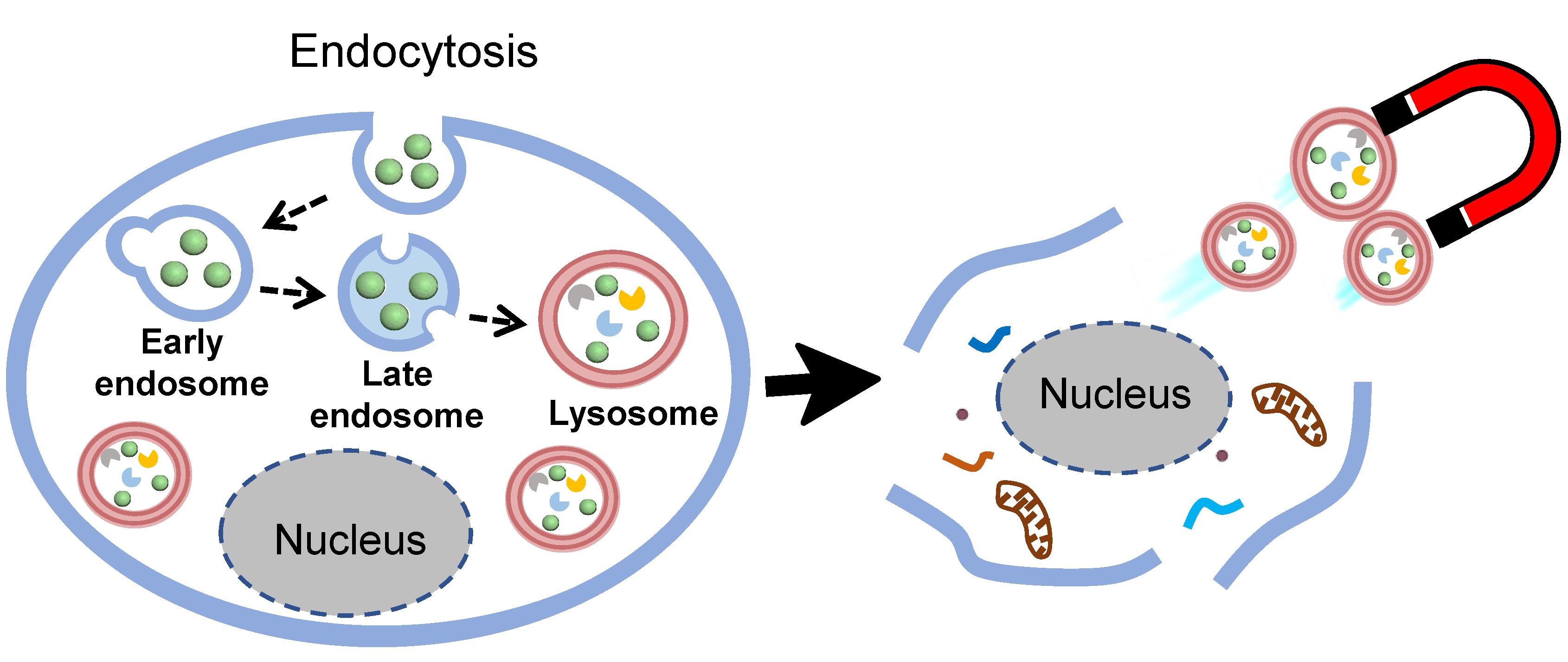
Background
Since their discovery by Christian de Duve in the 1950s (De Duve et al., 1955), the role of lysosomes in cellular function has been explored extensively, which led to the change of the view of lysosomes from a static digestive system, to the dynamic regulator of cellular metabolism. As indicated in various studies, lysosomal dysfunctions are found to be linked with the group of metabolic disorders known as lysosomal storage diseases (Mukherjee et al., 2019). Therefore, understanding lysosomal biology in both normal and pathogenic conditions is crucial to figuring out the mechanistic insights of lysosomal activity, to facilitate diagnostic methods, or establish a new therapeutic strategy.
The rapid and efficient isolation of lysosomes is a prerequisite to identify lysosomal protein composition, using proteomic analysis to reveal their involvement in cellular functions or disease progression. So far, several strategies have been developed to isolate lysosomes, including density-gradient centrifugation, immunoaffinity purification, and magnetic nanoparticle-based fractionation. Among these approaches, a nanoparticle-based method that delivers magnetic nanoparticles to the lumen of lysosomes, through an endocytic pathway, followed by a separation process, using a magnetic column, has been proven to be able to isolate lysosomes with the highest yield and purity, while efficiently preserving their integrity (Singh et al., 2020).
The accurate understanding of intracellular trafficking of magnetic nanoparticles is a key step to prevent contamination by other organelles (i.e., endosomes) in the magnetic nanoparticles-based fractionation of lysosomes. Generally, SPIONs are used as magnetic probes, which generally requires employing fluorescent dye-based techniques to monitor their intracellular trafficking. However, it has been suggested that the lysosomal environment could lead to quenching and/or distortion of fluorescence dye signals, which may cause an ensuing effect on the interpretation of the data (Milosevic et al., 2017). In addition, the surface modification of nanoparticles with dye molecules may influence the nano-bio interactions, which results in the alteration of the cellular uptake and intracellular trafficking of nanoparticles (Snipstad et al., 2017; Thomsen et al., 2021). Herein, to further refine the magnetic nanoparticle-based fractionation, the magnetic-plasmonic Ag/FeCo/Ag core/shell/shell nanoparticles (MPNPs) are used as multifunctional probes for lysosome isolation. Owing to their plasmonic properties, the intracellular trafficking of MPNPs can be easily investigated using confocal laser scanning microscopy, to confirm the accumulation of MPNPs in lysosomes, prior to magnetic isolation.
This protocol outlines the optimized procedures for preparation of MPNPs, intracellular trafficking study of MPNPs, and magnetic isolation of lysosomes. The time required for completing magnetic isolation of lysosomes after cell homogenization is within 30 min, which is significantly shorter than that of the density-gradient centrifugation technique. The amount of protein obtained was sufficient for mass spectroscopy, to identify protein composition. More importantly, this protocol was demonstrated to be easily adaptable to other adherent cell lines (Le et al., 2022).
Materials and Reagents
Glass syringe with lock tip 2 mL (Cadence Science, Stock Keeping Unit: 2407)
Glass syringe with lock tip 5 mL (Cadence Science, Stock Keeping Unit: 2417)
Stainless steel 304 syringe needle, noncoring point 2 inch 12G (Sigma-Aldrich, catalog number: Z116947-1EA)
Stainless steel 304 syringe needle, noncoring point 6 inch 20G (Sigma-Aldrich, catalog number: Z102709-1EA)
Centrifuge tube 50 mL (AS One, catalog number: 2-3939-03)
Microtube 1.5 mL (AS One, L-2057)
VIOLAMO 5 mL tube (AS One, catalog number: 2-4118-01)
Centrifuge tube 15 mL (AS One, catalog number: 1-3500-21)
Round cover glass Φ12mm No.1 (Matsunami, catalog number: C012001)
White slide glass edge grinding S1111 (AS One, catalog number: 2-154-01)
Terumo syringe with needle 2.5 mL 23G blue (AS One, catalog number: 1-2044-03)
Parafilm membrane (Amcor, Parafilm M, catalog number: PM996)
CELLect® Fetal bovine serum, 500 mL (FBS; MP Biomedicals, catalog number: 2917354H)
High-purity Ar gas, >99.9999 vol.%
Cobalt (II) acetylacetonate, 97% (Co precusor; Sigma-Aldrich, catalog number: 227129-50G)
Iron (III) acetylacetonate, 99.99% (Fe precusor; Sigma-Aldrich, catalog number: 517003-50G)
Silver nitrate, 99.9999% (Ag precusor; Sigma-Aldrich, catalog number: 204390-10G)
1,2-hexadecanediol, 90% (Sigma-Aldrich, catalog number: 213748-50G)
Oleylamine, 70% (Sigma-Aldrich, catalog number: O7805-500G), stored at 4°C
Oleic acid, 90% (Sigma-Aldrich, catalog number: 364525-1L), stored at 4°C
Tetraethylene glycol (Sigma-Aldrich, catalog number: 110175-1KG)
Acetone, 99.5% (Kanto Chemical, catalog number: 01026-70)
Hexane, 96% (Kanto Chemical, catalog number: 18041-70)
Chloroform, 99% (Kanto Chemical, catalog number: 07278-70)
Toluene, 99% (Wako Pure Chemical, catalog number: 201-01871)
1,2-dioleoyl-sn-glycerol-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-350] (PEG350-DOPE; Avanti, catalog number: 880430O-25MG), stored at −20°C
1,2-dioleoyl-sn-glycerol-3-phosphoethanolamine-N-(glutaryl) (18:1 Glutaryl PE; Avanti, catalog number: 870242C-25MG), stored at -20°C
2-morpholinoethanesulfonic acid, monohydrate, (MES; Dojindo, catalog number: 341-01622)
N-hydroxysuccinimide (NHS; Thermo Fisher Scientific, catalog number: 24500), stored at 4°C
Ethyl-3-(3-dimethyl aminopropyl) carbodiimide (EDC; Dojindo, catalog number: 346-03632), stored at 4°C
Amino dextran, MW. 10,000 (aDxt; Thermo Fisher Scientific, catalog number: D1860), stored at 4°C
Dulbecco’s phosphate buffer (PBS; Nissui Pharmaceutical, catalog number: 05913), stored at 4°C
Dulbecco’s modified Eagle’s medium (DMEM; Nacalai Tesque, catalog number: 08456-36), stored at 4°C
COS-1 cells (available from American Type Culture Collection, catalog number: CRL-1650)
Poly-L-lysine (PLL) solution, 0.01% (Sigma-Aldrich, catalog number: P4832-50ML)
4%-paraformaldehyde phosphate buffer, 500 mL (PFA; Nacalai Tesque, catalog number: 09154-85), stored at 4°C
Digitonin (Wako Pure Chemical, catalog number: 043-21376), stored at 4°C
Ammonium chloride (NH4Cl, Wako Pure Chemical, catalog number: 015-02991)
Bovine serum albumin (BSA; Sigma-Aldrich, catalog number: A8022-50G), stored at 4°C
Alexa Fluor® 647 mouse anti-human CD107A (AF647@CD107A; BD Biosciences, catalog number: 562622), stored at 4°C
4’,6-diamidino-2-phenylindole (DAPI; Thermo Fisher Scientific, catalog number: D1306), stored at 4°C
VECTASHIELD® Antifade mounting medium (Vector Laboratories, catalog number: H-1700), stored at 4°C
MES buffer (0.1 M, pH ~6) (see Recipes)
PBS buffer (see Recipes)
Digitonin solution (see Recipes)
Ammonium chloride solution (see Recipes)
DAPI staining solution (see Recipes)
Protease inhibitor solution (PIS) (see Recipes)
Note: The specific storage temperatures are indicated. Otherwise, chemicals are stored at room temperature (RT).
Equipment
Analytical balance (Sartorius, model: ME253P)
Three-neck round bottom flask 50 mL with angled side necks, center joint: ST/NS 29/42, side joints: ST/NS 15/25 (Tokyo Garasu Kikai, catalog number: 371-13-06-01)
Strong magnetic stirrer oval Φ12 × 25 mm (AS One, catalog number: 4-2687-04)
Laboran screw tube bottle 13.5 mL (glass vials; AS One, catalog number: 9-852-06)
Liebig condenser 300 mm, bottom joint: 29/42, top joint: 19/38 (Tokyo Garasu Kikai, catalog number: 330-15-51-14)
Digital high accuracy temperature controller (AS One, TJA-550, catalog number: 1-6124-01)
Mantle heater 50 mL (Tokyo Technological Labo, model: S-05)
High power stirrer (AS One, HPS-100, catalog number: 1-4136-01)
Flowmeter (Kofloc, model: RK1250)
Septum rubber, white, natural, for 18 mm tube (FUJIFILM Wako Pure Chemical, catalog number: 195-11771, Japanese Article Number: 4987481378957)
FisherbrandTM Pasteur pipets (Fisher Scientific, catalog number: 22-063156)
Double element thermocouple WK-Φ3.2×200 (AS One, catalog number: 3-9391-14)
Trap sphere, top and bottom joints: 29/42 (Tokyo Garasu Kikai, catalog number: 330-15-91-07)
Refrigerated centrifuge (Kubota, model: 5910 (with RS-410M rotor))
Ultraviolet-visible absorption spectrophotometer (JASCO, model: V-750)
Two-neck round bottom flask 50 mL with an angled side neck, center joint, and side joints: 14/24
TS one-neck round bottom flask 100 mL,15/25, with the glass stopper (Climbing Co., ltd., CL0070-05-11)
Sonicator (AS One, Ultrasonic Cleaner ASU-6, oscillation frequency: 40 kHz)
High-speed micro centrifuge (Hitachi Koki, model: Himac CF15RXII (with T16A31 rotor))
Ultracentrifuge (Eppendorf Himac Technologies, model: CS100FNX (with S100AT4-2004 rotor))
37°C and 5% CO2 incubator (ESPEC, model: BNA-111)
Confocal laser scanning microscope (CLSM; Olympus, model: FV1000D)
Cell Lifter (Corning, product number: 3008)
MidiMACS separator starting kits (Miltenyi Biotec, catalog number: 130-042-301)
MS Column (Miltenyi Biotec, catalog number: 130-042-201)
High-speed refrigerated micro centrifuge [Tomy Seiko, model: MDX-310 (with AR015-24 rotor)]
Software
Fiji (NIH/https://imagej.net/software/fiji/), with color clustering and coloc 2 plugins
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Le, T. S., Takahashi, M. and Maenosono, S. (2022). A Robust Nanoparticle-based Magnetic Separation Method for Intact Lysosomes. Bio-protocol 12(13): e4453. DOI: 10.21769/BioProtoc.4453.
分类
生物工程 > 生物医学工程
细胞生物学 > 细胞器分离 > 溶酶体
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link