- EN - English
- CN - 中文
Ex vivo Human Skin Infection with Herpes Simplex Virus 1
人皮肤单纯疱疹病毒1的体外感染
发布: 2022年05月05日第12卷第9期 DOI: 10.21769/BioProtoc.4411 浏览次数: 3027
评审: Vasudevan AchuthanThirupugal GovindarajanAnonymous reviewer(s)

相关实验方案
诱导型HIV-1库削减检测(HIVRRA):用于评估外周血单个核细胞中HIV-1潜伏库清除策略毒性与效力的快速敏感方法
Jade Jansen [...] Neeltje A. Kootstra
2025年07月20日 2449 阅读
Abstract
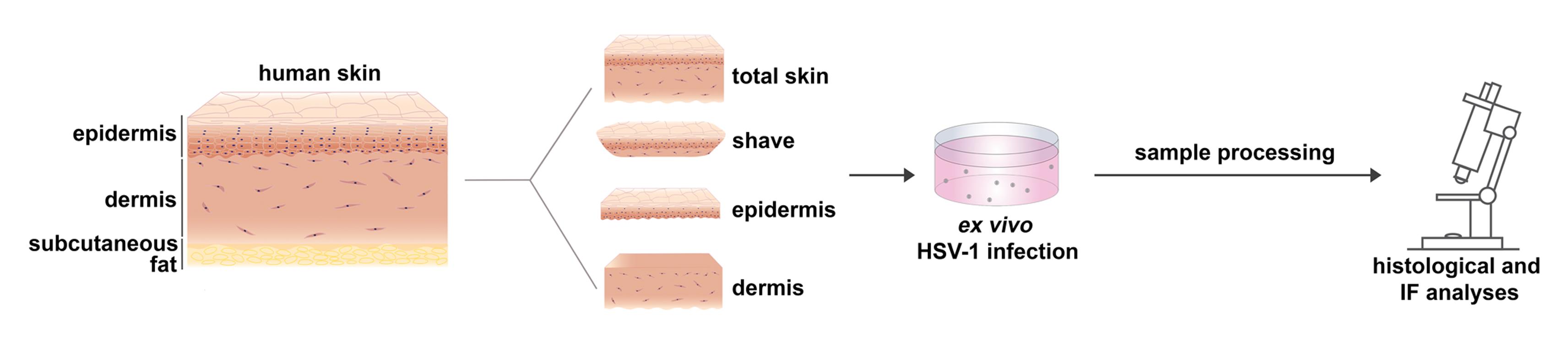
Although herpes simplex virus 1 (HSV-1) is a well-studied virus, how the virus invades its human host via skin and mucosa to reach its receptors and initiate infection remains an open question. For studies of HSV-1 infection in skin, mice have been used as animal models. Murine skin infection can be induced after injection or scratching of the skin, which provides insights into disease pathogenesis but is clearly distinct from the natural entry route in human tissue. To explore the invasion route of HSV-1 on the tissue level, we established an ex vivo infection assay using skin explants. Here, we detail a protocol allowing the investigation of how the virus overcomes mechanical barriers in human skin to penetrate in keratinocytes and dermal fibroblasts. The protocol includes the preparation of total skin samples, skin shaves, and of separated epidermis and dermis, which is followed by incubation in virus suspension. The ex vivo infection assay allows the visualization, quantification, and characterization of single infected cells in the epidermis and dermis prior to viral replication and the virus-induced tissue damage. Hence, this experimental approach enables the identification of primary viral entry portals.
Graphical abstract:
Background
Herpes simplex virus 1 (HSV-1) is one of the most prevalent human pathogens. During primary infection, the virus invades skin and mucocutaneous sites, which is followed by the latent infection of sensory neurons. Upon reactivation, HSV-1 travels back to near the initial infection site and can cause lesions. As the skin and mucosa form a protective barrier to infection, the general assumption is that the virus infects its host organism via epithelial breaks due to mechanical injuries or pathophysiological modifications. Viral replication largely takes place in the epithelium, and the extent of productive infection is restricted by the immune response. How HSV-1 enters single cells in culture is extensively studied; however, we know less about the viral invasion in human skin and mucosa to initiate infection in skin cells. Thus, the conditions under which the virus overcomes the epithelial barriers, either during primary or recurrent infection, and gains access to its receptors on the skin cells in vivo, are yet to be elucidated.
To address HSV-1 infection in tissue, initial studies were performed in 3-dimensional (3D) skin cultures giving first insights into how the virus may spread and how efficiently differentiating human keratinocytes can be infected (Syrjänen et al., 1997; Visalli et al., 1997; Hukkanen et al., 1999). More recently, human mucosa and skin explants have been used as ex vivo HSV-1 infection models, which allow studying various steps of viral infection much closer to the in vivo situation than 3D cultures or animal models. To analyze the determinants of viral invasion, we ex vivo infected human oral mucosa and detected no infected cells in complete mucosa samples but only invasion in the basal epithelial layer once the connective tissue and the basement membrane were removed (Thier et al., 2017). Intriguingly, mechanical wounding of the mucosa samples is insufficient for HSV-1 to overcome the epithelial barriers (Thier et al., 2017). However, successful HSV-1 infection was observed in human abdominal skin after ex vivo infection of microneedle-pretreated skin, thus providing a model to study the efficiency of antiviral drugs (Tajpara et al., 2018). Tajpara and colleagues (2018) infected thin dermatome-cut skin with lesions partly penetrating both epidermis and dermis and showed the pathogenesis of HSV-1 4 days after infection. The assumption is that viral invasion occurs via the sample edges and at those lesions that cross the skin sample, which, in turn, leads to extensive tissue damage over time. To analyze the initial sites of HSV1 invasion in human skin upon wounding and address the susceptibility of the epidermal layers and the dermis, we developed the protocol described here (De La Cruz et al., 2021). The procedure is based on our ex vivo infection studies in murine skin and human oral mucosa (Rahn et al., 2015a, 2015b; Thier et al., 2017; Wirtz et al., 2020; De La Cruz and Knebel-Mörsdorf, 2021). Our focus is on the early events of infection to understand how the virus overcomes the distinct epidermal barriers, identify which cells are initially infected, and determine how the virus spreads in tissue. As murine and human total skin samples are protected against ex vivo infection from the apical site confirming the protective barrier of healthy skin (Rahn et al., 2015b; De La Cruz et al., 2021), we dissected the skin samples to investigate how susceptible the epidermis and dermis are. After separation of the dermis from the epidermis by dispase II treatment, the basement membrane is disrupted, and remaining components are only occasionally found on the apical site of the dermis and the basal layer of the epidermis. Infection of the separated human epidermis with high virus dose leads to infection of basal and suprabasal keratinocytes, which results in cytopathic effects at 24 h postinfection (p.i.) (De La Cruz et al., 2021). The onset of viral infection in single basal keratinocytes and viral spreading in suprabasal layers can be visualized by immunostaining of very early expressed viral genes such as ICP0 and ICP4. Alternatively, virus particles could be stained or tagged HSV-1 strains could be used for infection. The challenge is to visualize single particles in tissue prior to viral gene expression, to differentiate between noninfectious and infectious particles, and to localize particles in- or outside of cells. As the human epidermis is highly susceptible to HSV 1, virus titers can be determined at various times p.i. In contrast to the epidermis, the infection protocol results only in single infected fibroblasts in the human dermis even at 24 h p.i. (De La Cruz et al., 2021).
In addition, the skin samples could be mechanically wounded or stimulated with cytokines prior to ex vivo infection. Further modifications of the skin surface could be achieved by pre-incubation with bacterial or viral pathogens.
Our infection protocol is most suitable for investigating the early steps of HSV-1 infection in tissue. These include the interaction with epidermal barriers and with components of the extracellular matrix, the role of cellular receptors that allow viral penetration, and the cellular response to initial infection. The dissection of the human skin in skin shaves, epidermis, and dermis offers various experimental tools to address questions from different perspectives.
Materials and Reagents
Feather® sterile disposable scalpels, stainless steel blade with plastic handles (blade No. 21)
Suture/surgical scissors (from any qualified supplier)
Filtered micropipette tips (Sapphire, various sizes, e.g., 10 μL, 300 μL, and 1,250 μL, Greiner, catalog numbers: 772363, 738265, 750265)
5 and 10 mL serological pipettes (Greiner, catalog number: 606 180)
50 mL conical centrifuge tubes (Greiner, catalog number: 227261)
Syringe (various sizes, e.g., 5–20 mL from any qualified supplier)
Millex® disposable syringe filter units, disposable 0.22 μm pore size (Merck Chemicals, catalog number: SLGP033RK)
40 μm cell strainers (Corning, catalog number: 352340)
Dispase II (Roche, catalog number 4942078001), stored at 4°C
Tissue culture plates, 24-well, flat bottom (Corning Incorporated, catalog number: 3524)
Tissue culture plates, 6-well, flat bottom (Corning Incorporated, catalog number: 3516)
DMEM-high glucose-GlutaMAX (GIBCO, catalog number: 10566016), stored at 4°C
Fetal calf serum (same batch of FCS used for a complete series of experiments to avoid variable effects of FCS components)
Penicillin/streptomycin solution (from any qualified supplier)
formaldehyde solution (37%) (Sigma-Aldrich, catalog number: 8187081000), store at RT, prepare working solution freshly
Peel-A-Way embedding Molds, truncated-T8 (Polysciences, catalog number: 18985-1)
Tissue embedding and processing cassettes (from any qualified supplier)
Sakura FinetekTM Tissue-TekTM O.C.T. Compound (ThermoFisher Scientific, catalog number: 12351753)
Paraffin No. 3/6/9 (Richard-Allan-Scientific, ThermoFisher Scientific, catalog number: 8335)
phosphate buffered saline (PBS, sterile) (from any qualified supplier)
Korsolex® basic (BODE Chemie, catalog number: 0022014) for instrument disinfection
Wt HSV-1 (Glasgow strain 17+); purified from the supernatant of infected BHK cells via density gradient centrifugation (5–15% ficoll gradients); virus titer determined by plaque assays in Vero-B4 cells (see Grosche et al., 2019)
whole-skin dissociation kit (Miltenyi biotec, catalog number: 130-101-540)
DPX mounting medium (Sigma-Aldrich, catalog number: 06522)
TryplE Select (GIBCO, catalog number: 12563029)
Cell Dissociation Solution Non-enzymatic 1× (Sigma-Aldrich, catalog number: C5914)
Cell culture medium (see Recipes)
Epidermal cryosection blocking buffer (see Recipes)
Dermal and total (full-thickness) skin cryosection blocking buffer (see Recipes)
Dispase II solution (see Recipes)
Whole Mount Blocking Buffer (see Recipes)
PBS-Tween (see Recipes)
Equipment
Precision forceps (curved and straight) (from any qualified supplier)
5% CO2 incubator, set at 37°C
Paraffin microtome
Cryostat
Class II biosafety cabinet
Fume hood
FACS machine (Canto II BD Biosciences)
Software
ImageJ-win64 (open source; Schneider et al., 2012)
FacsDIVA v6.1.3 (BD)
FlowJo v7.6.3 (Tree Star)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
De La Cruz, N. C., Möckel, M., Wirtz, L. and Knebel-Mörsdorf, D. (2022). Ex vivo Human Skin Infection with Herpes Simplex Virus 1. Bio-protocol 12(9): e4411. DOI: 10.21769/BioProtoc.4411.
分类
微生物学 > 微生物-宿主相互作用 > 离体模型
微生物学 > 微生物-宿主相互作用 > 病毒
细胞生物学 > 组织分析 > 组织成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link