- EN - English
- CN - 中文
Identification of SARS-CoV-2 Neutralizing Antibody with Pseudotyped Virus-based Test on HEK-293T hACE2 Cells
基于 HEK-293T hACE2 细胞的假型病毒试验鉴定 SARS-CoV-2 中和抗体
发布: 2022年04月05日第12卷第7期 DOI: 10.21769/BioProtoc.4377 浏览次数: 2636
评审: Juan Facundo Rodriguez AyalaLuis Alberto Sánchez VargasAnonymous reviewer(s)

相关实验方案

用于检测和定量人血浆或血清中抗病毒抗体的优化间接 ELISA 方案:以 SARS-CoV-2 为例的案例研究
Claire Baine [...] Jennifer Serwanga
2023年12月20日 3649 阅读

诱导型HIV-1库削减检测(HIVRRA):用于评估外周血单个核细胞中HIV-1潜伏库清除策略毒性与效力的快速敏感方法
Jade Jansen [...] Neeltje A. Kootstra
2025年07月20日 2460 阅读
Abstract
Neutralizing antibodies (NAbs) are of particular importance because they can prevent binding of the receptor binding domain (RBD) of the spike protein (S protein) to the angiotensin-converting enzyme 2 (ACE2) receptor present at the surface of human cells, preventing virus entry into the host cells. The gold standard method for detection of NAbs is the plaque reduction neutralization test (PRNT). Based on the measurement of cell lysis due to viral infection, this test is able to detect antibodies that prevent cell infection (Muruato et al., 2020; Lau et al., 2021). This technique requires the use of live pathogens, i.e., SARS-CoV-2 in this case, and must be done in a biosafety level 3 (BL3) laboratory. In addition, it requires expensive installations, skillful and meticulous staff, and a high workload, which prevents its wide implementation even in research laboratories. A SARS-CoV-2 pseudovirus will express the S protein responsible for cell entrance, but will not express the pathogenic genetic material of the virus, making them less dangerous for laboratory staff and the environment.
Graphic abstract:
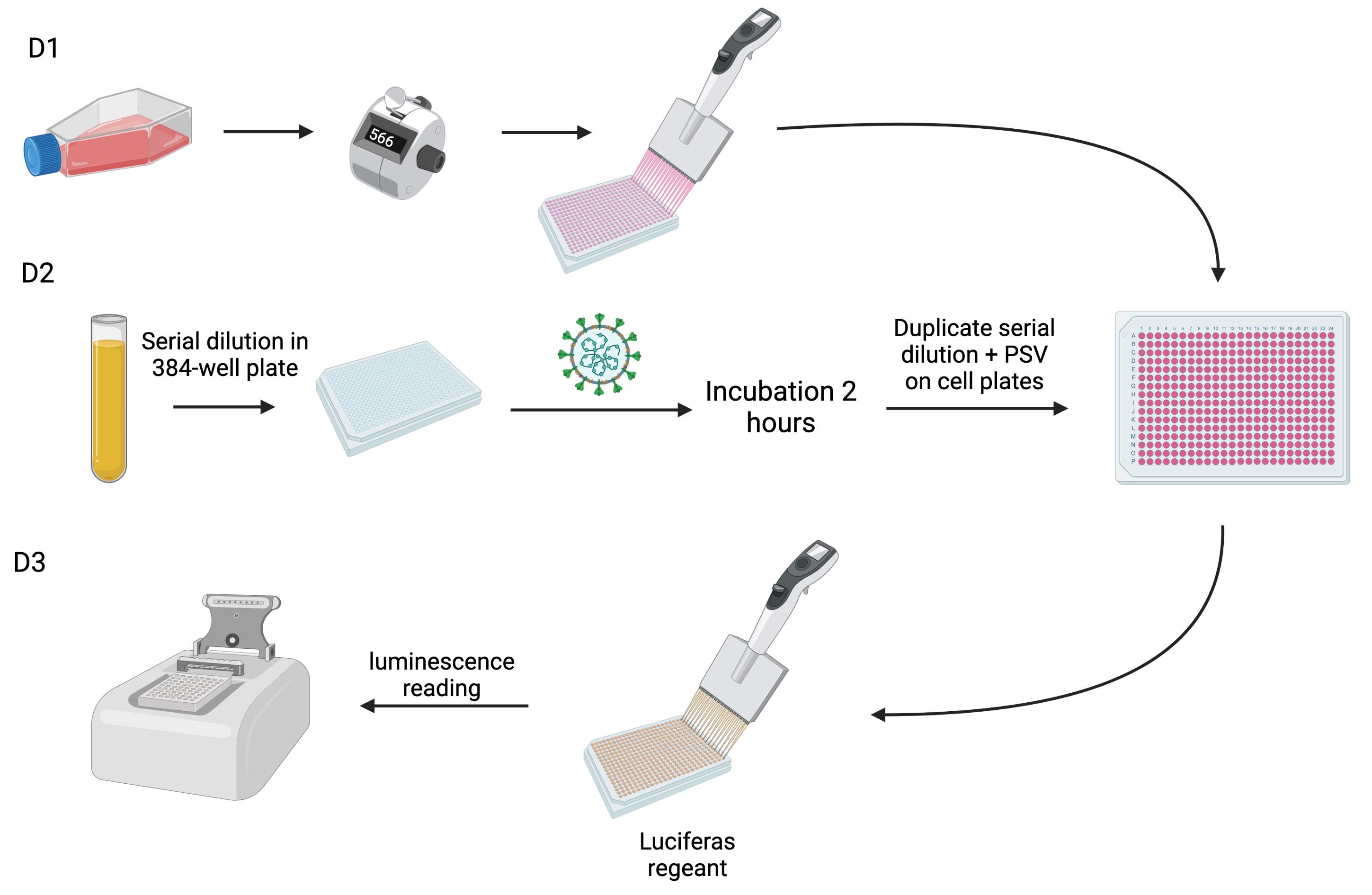
Background
The gold standard method for detection of NAbs is the plaque reduction neutralization test (PRNT) (Perera et al., 2020). Based on the measurement of cell lysis due to viral infection, this test is able to detect antibodies that prevent the cell infection (Muruato et al., 2020; Lau et al., 2021). Such technique requires the use of live pathogens, i.e., SARS-CoV-2 in this case, and must be done in a biosafety level 3 (BL3) laboratory. In addition, it requires expensive installations, skillful and meticulous staff, and a high workload, which prevent its wide implementation even in research laboratories (Muruato et al., 2020; Lee et al., 2021). Such facilities are not widely available, and only very specialized institutions can offer access to BL3 laboratories and trained staff. Quite similar neutralization techniques based on pseudoviral particles (called pseudo-virus neutralization tests, pVNT) have been developed, and can be performed in BL2 laboratories, allowing higher throughput (Nie et al., 2020a). A SARS-CoV-2 pseudovirus will express the S protein responsible for cell entrance, but will not express the pathogenic genetic material of the virus, making them less dangerous (Nie et al., 2020a, 2020b).
Materials and Reagents
Sterile white 384-well µClear flat bottom cell culture plate with lid (Greiner Bio-One, Kremsmünster, Austria, catalog number: 781098)
Sterile 384-well flat bottom assay plate with lid (Corning, NY, USA, catalog number: 3701)
Pipette tip 200 µL (Thermo Fisher Scientific, Waltham, MA, USA, catalog number: AM12650)
Eppendorf tube (Sigma-Aldrich, Saint-Louis, MO, USA, catalog number: T2795)
50 mL reagent reservoir sterile polystyrene (Merck, Overijse, Belgium, catalog number: CLS4870)
HEK-293T hACE2 (Invivogen, San Diego, CA, USA, catalog number: HKB-hACE2)
SARS-CoV-2 Pseudoviral Particles (E-enzyme, Gaithersburg, MD, USA, catalog number: SCV2-PsV-001)
Dulbecco’s Modified Eagle Medium (DMEM), with L-glutamine and glucose (Lonza, Bâle, Switzerland, catalog number: LO BE12-604F)
FireFly Luciferase kit (E-enzyme, Gaithersburg, MD, USA, catalog number: CA-L165-10)
Tryptan blue (Invitrogen by Thermo Fisher Scientific, Waltham, MA, USA, catalog number: T10282)
Equipment
Spectramax 3 iD (Molecular Devices, LLC, CA, USA)
Laminar flow hood (Thermo Fisher Scientific, Waltham, MA, USA, MSC Advantage 1.8 catalog number: 51025413)
Electronic multichannel 5–125 µL pipette (Brand, Transferpette -12 electronic, catalog number: 705453)
Monochannel 5–50 µL pipette (Socorex, Ecubens, Switzerland, catalog number: 825.0050)
Centrifuge 5702 (Eppendorf, Hamburg, Deutschland, catalog number: 5702000320)
Neubauer counting slide (Hecht Assistant, Altnau, Switzeland, catalog number: 40441)
Julobo ED Water bath (Sigma Aldrich, Saint-Louis, MO, USA, catalog number: Z615498)
Software
GraphPad Prism software (version 9.1.0, San Diego, CA, USA)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Gillot, C., Favresse, J., Maloteau, V., Dogné, J. M. and Douxfils, J. (2022). Identification of SARS-CoV-2 Neutralizing Antibody with Pseudotyped Virus-based Test on HEK-293T hACE2 Cells. Bio-protocol 12(7): e4377. DOI: 10.21769/BioProtoc.4377.
分类
免疫学 > 抗体分析 > 抗体检测
医学
细胞生物学 > 基于细胞的分析方法 > 病毒性感染
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










