- EN - English
- CN - 中文
Rapid Lipid Quantification in Caenorhabditis elegans by Oil Red O and Nile Red Staining
油红 O 和尼罗红染色对秀丽隐杆线虫的快速脂质定量
(*contributed equally to this work) 发布: 2022年03月05日第12卷第5期 DOI: 10.21769/BioProtoc.4340 浏览次数: 8327
评审: Manish ChamoliAnupama SinghAnnmary Paul ErinjeriAnand Ramesh Patwardhan
Abstract
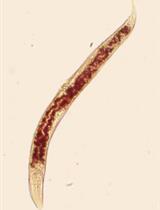
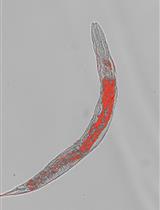
The ability to stain lipid stores in vivo allows for the facile assessment of metabolic status in individuals of a population following genetic and environmental manipulation or pharmacological treatment. In the animal model Caenorhabditis elegans, lipids are stored in and mobilized from intracellular lipid droplets in the intestinal and hypodermal tissues. The abundance, size, and distribution of these lipids can be readily assessed by two staining methods for neutral lipids: Oil Red O (ORO) and Nile Red (NR). ORO and NR can be used to quantitatively measure lipid droplet abundance, while ORO can also define tissue distribution and lipid droplet size. C. elegans are a useful animal model in studying pathways relating to aging, fat storage, and metabolism, as their transparent nature allows for easy microscopic assessment of lipid droplets. This is done by fixation and permeabilization, staining with NR or ORO, image capture on a microscope, and computational identification and quantification of lipid droplets in individuals within a cohort. To ensure reproducibility in lipid measurements, we provide a detailed protocol to measure intracellular lipid dynamics in C. elegans.
Graphic abstract:

Flow chart depicting the preparation of C. elegans for fat staining protocols.
Background
In Caenorhabditis elegans, lipid homeostasis is one of the many cellular processes that declines with age (Lynn et al., 2015; Johnson and Stolzing, 2019, Hammerquist et al., 2021). C. elegans are a popular model organism for many reasons, such as rapid generation time, short lifespan, determinate cell fate, and the availability of a fully sequenced genome, in addition to the myriad genetic and molecular tools that have been developed and optimized for use in this system. One such area in which C. elegans are particularly useful is the study of lipid metabolism, because of the multiple techniques that have been developed to determine lipid content in worms (Zhang et al., 2013). Analysis of specific lipid species can be conducted with the use of high-performance liquid chromatography-mass spectrometry and gas-chromatography-mass spectrometry (Castro et al., 2012; Pino et al., 2013). Although useful in determining lipid extract complexity, these methods require time for analysis and expensive machinery that isn’t readily available for all laboratories. Here, we describe two low-cost methods that take advantage of the transparency of the worm for visualizing the amount and tissue distribution of intracellular neutral lipids and require no more specialized equipment than present in most laboratories equipped to study C. elegans. C. elegans can be stained with many lipophilic dyes; two of the most readily available, easy-to-use, and biochemically validated compounds are Oil Red O (ORO) and Nile Red (NR) (Pino et al., 2013; Pang and Curran, 2014; Lynn et al., 2015; Webster et al., 2017; Stuhr and Curran, 2020). NR or 9-diethylamino-5H-benzo[α]phenoxazine-5-one is a lipophilic dye that stains intracellular neutral lipid droplets (Greenspan et al., 1985). NR allows for the quantification of lipid abundance due to the emission of green light following excitation. ORO is a fat-soluble dye that stains neutral lipids a bright red color, allowing for qualitative assessment of lipid droplet size and lipid distribution (O'Rourke et al., 2009). Staining C. elegans with these two different dyes allows for the determination of alterations in quantity and distribution of neutral lipids.
Materials and Reagents
Sterile 6 cm Petri dishes (VWR, catalog number: 25373-085)
Platinum wire (Tritech Research, catalog number: PT-9010)
200 μL micropipette tips (Genesee Scientific, catalog number: 24-151RL)
1,000 μL micropipette tips (Genesee Scientific, catalog number: 23-165R)
15 mL centrifuge tubes with EZ flip cap (ThermoFisher Scientific, catalog number: 362694)
1.5 mL microtube (Axygen Scientific, catalog number: MCT-150-C)
Parafilm (Sigma-Aldrich, catalog number: P7543)
10 mL disposable syringe (VWR, catalog number: 76290-382)
0.2 μm syringe filters (VWR, catalog number: 28145-477)
Eyelash brush (consisting of a human eyelash attached to a Pasteur pipette, generic, with tape, generic)
Microscope slides (VWR, catalog number: 48312-004)
Microscope slide cover slips (VWR, catalog number: 48366-227)
Razor blade, generic
LB powder (Teknova, catalog number: L9315)
Magnesium sulfate (MgSO4) (Sigma-Aldrich, catalog number: M2773)
Cholesterol (Sigma-Aldrich, catalog number: C8667)
Calcium chloride (CaCl2) (Sigma-Aldrich, catalog number: C3881)
Streptomycin sulfate (Sigma-Aldrich, catalog number: S6501)
Sodium chloride (NaCl) (Fisher Scientific, catalog number: 02-004-047)
Bacto agar (BD, catalog number: 214040)
Peptone (BD, catalog number: 211820)
50% sodium hydroxide (Fisher Scientific, catalog number: SS254-500)
Clorox® disinfecting bleach with CLOROMAX®–Concentrated Formula (Clorox)
Oil Red O (Alfa Aesar, catalog number: A12989)
DAPI (Sigma-Aldrich, catalog number: D9542)
Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D2650-5X10ML)
Sodium phosphate dibasic (Na2HPO4) (Sigma-Aldrich, catalog number: S9763)
Potassium phosphate dibasic (K2HPO4) (Sigma-Aldrich, catalog number: P5504)
Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: P0662)
Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P3911)
Triton X-100 (Sigma-Aldrich, catalog number: X100-100 ml)
Isopropanol (BHD, catalog number: BDH1133)
Ethanol (VWR, catalog number: 89125-172)
Nail polish (clear, generic)
Nile Red for microscopy (Sigma-Aldrich, catalog number: 72485)
Acetone 99.5% (BDH, catalog number: 1101-1LP)
Nematode growth medium (NGM) plates (see Recipes)
M9 (see Recipes)
Oil Red O stock solution (see Recipes)
DAPI stock solution (see Recipes)
Phosphate buffered saline (PBS) (see Recipes)
PBS + 0.01% Triton X-100 (PBST) (see Recipes)
Nile Red stock solution (see Recipes)
Worm strains (available from Caenorhabditis Genetics Center)
E. coli OP50-1 (available from Caenorhabditis Genetics Center)
Equipment
2–20 μL micropipette (Gilson, catalog numer: FA10003M)
20–200 μL micropipette (Gilson, catalog numer: FA10005M)
100–1,000 μL micropipette (Gilson, catalog numer: FA10006M)
Microcentrifuge for 1.5 mL tubes (Eppendorf, model: 5430)
Centrifuge for 15 mL tubes (Eppendorf, model: 5702)
Safe Aspiration Station (Gilson, model: F110745)
Worm incubator (Generic, maintain at 20°C)
Tube rotator (Thermo Scientific, catalog number: 88881001)
Compound microscope with DIC, DAPI, and GFP filters, and 5× and 10× objectives (Zeiss, model: AxioScope5)
Color camera (Zeiss AxioCam MRm)
Digital camera (Zeiss AxioCam ERc5s)
Stir plates, generic
Magnetic stir bars, generic
Software
Software for image acquisition
Dependent on the microscope used (in this case, we used a Zeiss Axioscope and the associated ZEN imaging software).
ImageJ (available from NIH) for image quantification
Data analysis software
We used GraphPad prism, although any software capable of t-test will suffice. ANOVA may be helpful if comparing multiple conditions, but not strictly necessary. For a pairwise comparison, a t-test can be conducted, while three or more comparisons will require an ANOVA.
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Stuhr, N. L., Nhan, J. D., Hammerquist, A. M., Van Camp, B., Reoyo, D. and Curran, S. P. (2022). Rapid Lipid Quantification in Caenorhabditis elegans by Oil Red O and Nile Red Staining. Bio-protocol 12(5): e4340. DOI: 10.21769/BioProtoc.4340.
分类
生物化学 > 脂质 > 脂质测定
细胞生物学 > 细胞染色 > 脂质
细胞生物学 > 细胞新陈代谢 > 脂质
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link