- EN - English
- CN - 中文
Skeletal Stem Cell Isolation from Cranial Suture Mesenchyme and Maintenance of Stemness in Culture
从颅缝间充质中分离骨骼干细胞和培养干细胞的维持
发布: 2022年03月05日第12卷第5期 DOI: 10.21769/BioProtoc.4339 浏览次数: 3348
评审: Salma MerchantJunichi IwataValerie Uytterhoeven
Abstract
Skeletal stem cells residing in the suture mesenchyme are responsible for calvarial development, homeostatic maintenance, and injury-induced repair. These naïve cells exhibit long-term self-renewal, clonal expansion, and multipotency. They possess osteogenic abilities to regenerate bones in a cell-autonomous manner and can directly replace the damaged skeleton. Therefore, the establishment of reliable isolation and culturing methods for skeletal stem cells capable of preserving their stemness promises to further explore their use in cell-based therapy. Our research team is the first to isolate and purify skeletal stem cells from the calvarial suture and demonstrate their potent ability to generate bone at a single-cell level. Here, we describe detailed protocols for suture stem cell (SuSC) isolation and stemness maintenance in culture. These methods are extremely valuable for advancing our knowledge base of skeletal stem cells in craniofacial development, congenital deformity, and tissue repair and regeneration.
Keywords: Bone regeneration (骨再生)Background
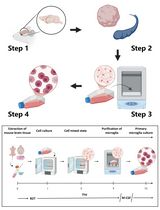
Conventional methods have shown successful isolation of mesenchymal stromal cells (MSCs) from bone marrow and other tissues (Friedenstein et al., 1974; da Silva Meirelles et al., 2006). MSCs contain skeletal stem cells (SSCs) with self-renewing and skeletogenic differentiating abilities (Sacchetti et al., 2007). However, only ~10–20% of them are genuine SSCs (Robey et al., 2014). The difficulties in engraftment, survival, and differentiation of the transplanted MSCs have also been well documented (Caplan and Correa, 2011; Zeitouni et al., 2012). Furthermore, the cellular source of the endogenous MSC remains unknown. Here, we describe a protocol for the isolation of SSCs from the calvarial suture mesenchyme. These SuSCs have been demonstrated to generate bone at a single cell level upon kidney capsule transplantation, which can faithfully assess SuSC properties in vivo (Maruyama et al., 2016). It is also important to develop a protocol capable of maintaining their stemness in vitro. The successful establishment of the skeletal stem cell culture protocol permits further assessments of stem cell characteristics, e.g., self-renewal, proliferation, fate determination, and differentiation in an ex vivo setting. The preservation of stem cell stemness in culture is also critical for bone tissue engineering and opens the possibility of exploring next-generation therapeutics. We also describe an ex vivo protocol to culture SuSCs for an extended period. The cultured SuSCs can generate bones upon implantation to the ectopic site (Maruyama et al., 2021). We demonstrate that this culture method can determine the label-retaining ability and asymmetric division of SuSCs. It also provides an outstanding system to further our examination of additional stem cell characteristics, e.g., cell fate determination, generation of skeletal progenitors, and skeletogenic differentiation, as well as studies at the transcriptome (RNA-seq), epigenetics (ATAC-seq and ChIP-seq), and single-cell levels (scRNA-seq). Using these new tools, we can reconstruct lineage relationships between cells within craniofacial and skeletal tissues—a long-standing challenge in biology. These are extremely important advancements in stem cell-based therapy for bone regeneration and repair.
Materials and Reagents
Cell strainer 40 µm Nylon (Falcon, catalog number: 352340)
Ultra-Low Attachment Surface 24-well plate (Corning, catalog number: 3473)
Dulbecco’s Phosphate-Buffered Saline without calcium & magnesium (DPBS; Corning, catalog number: 21-031-CV), 2 years shelf life at room temperature
0.2% collagenase A (Roche, catalog number: 11088793001), freshly reconstituted from lyophilized stock in PBS
10 mM HEPES (1 M buffer solution; Gibco, catalog number: 15630-080), 24 months shelf-life at 4°C
Penicillin-Streptomycin (Gibco, catalog number: 15140-122), 12 months shelf life at -20°C
100 µg/mL transferrin (Sigma, catalog number: T8158) at -20°C
20 nM progesterone (Sigma, catalog number: P8783) at -20°C
30 nM Sodium selenite (Sigma, catalog number: S8295) at -20°C
60 nM Putrescine dihydrochloride (Sigma, catalog number: P5780), 4 years shelf life at -20°C
EGF (Mouse EGF Recombinant Protein; Gibco, catalog number: PMG8041), 1 year shelf life at -20°C
bFGF (mouse FGF-basic Recombinant Protein; Gibco, catalog number: PMG0035), 1 year shelf life at -20°C
B27 supplement (Gibco, catalog number: 17504044,), 1 year shelf life at -20°C
Insulin (Sigma, catalog number: I1882) at -20°C
0.25% trypsin (Gibco, catalog number: 25200-056), 2 years shelf life at -20°C
Soybean trypsin inhibitor (SBTI; Sigma, catalog number: T9128) at -20°C
Digestion Buffer (see Recipes)
Sphere Culture Media (see Recipes)
2× SBTI Solution (see Recipes)
Equipment
Shaker (Thermo Scientific, MaxQtm 4000 Benchtop Orbital Shakers)
Centrifuge (Thermo Scientific, Sorvall ST 16R Centrifuge)
Incubator (Thermo Forma, Series II Water Jacketed CO2 Incubator)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Maruyama, T., Yu, H. I. and Hsu, W. (2022). Skeletal Stem Cell Isolation from Cranial Suture Mesenchyme and Maintenance of Stemness in Culture. Bio-protocol 12(5): e4339. DOI: 10.21769/BioProtoc.4339.
- Maruyama, T., Stevens, R., Boka, A., DiRienzo, L., Chang, C., Yu, H. I., Nishimori, K., Morrison, C. and Hsu, W. (2021). BMPR1A maintains skeletal stem cell properties in craniofacial development and craniosynostosis. Sci Transl Med 13(583): eabb4416.
分类
干细胞 > 成体干细胞
细胞生物学 > 细胞分离和培养 > 细胞分离
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link