- EN - English
- CN - 中文
An in vitro Assay of mRNA 3’ end Using the E. coli Cell-free Expression System
使用大肠杆菌无细胞表达系统对 mRNA 3' 端进行体外测定
发布: 2022年02月20日第12卷第4期 DOI: 10.21769/BioProtoc.4333 浏览次数: 3675
评审: Emilia KrypotouSrujana Samhita YadavalliAnonymous reviewer(s)
Abstract
At the end of about 80% of the operon in Escherichia coli, translation termination decouples transcription, leading to Rho-dependent transcription termination (RDT). However, no in vitro or in vivo assay system has proven to be good enough to see the 3’ end of the mRNA generated by RDT. Here, we present a cell-free assay system that could provide detailed information on the 3’ end of a transcript RNA generated by RDT. Our protocol shows how to extract transcript RNA generated by transcription reactions from a cell-free extract, followed by an RNA oligomer ligation to the 3’ end of a transcript RNA of interest. The 3' end of the RNA is amplified using RT-PCR. Its genetic location can be determined using a gene-specific primer extension reaction. The 3’ ends of mRNA can be visualized and quantified by polyacrylamide gel electrophoresis. One significant advantage of a cell-free assay system is that factors involved in the generation of the 3' end, such as proteins and sRNA, can be directly assayed by exogenously adding factor(s) to the reaction.
Graphic abstract:
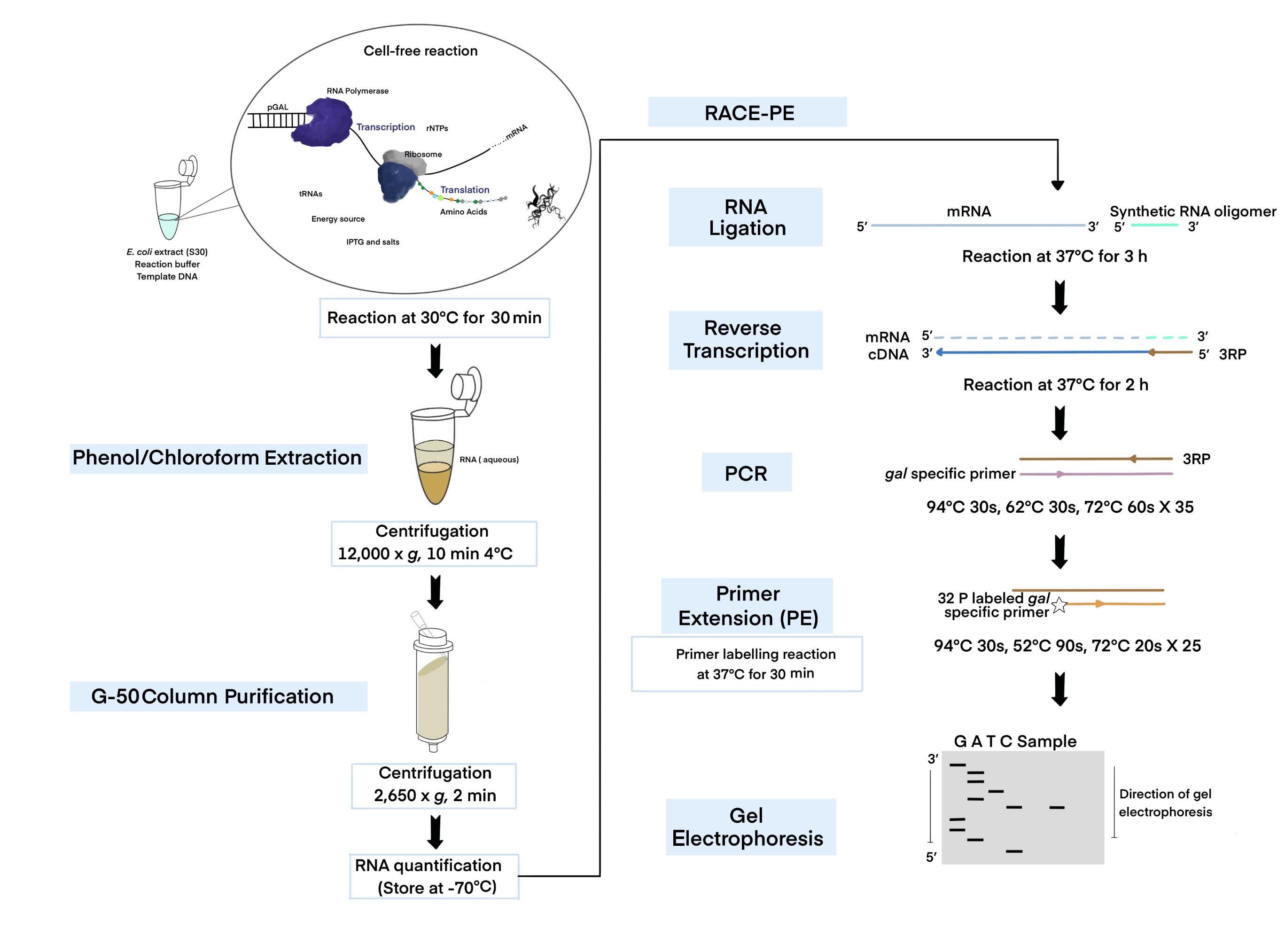
An illustration of the experimental methodology.
Background
In bacteria, there are two types of transcription termination: 1) intrinsic or Rho-independent termination (RIT), caused by the terminator hairpin formed on the transcript at the end of the last transcription unit, and 2) Rho-dependent termination (RDT) (Ray-Soni et al., 2016). In prokaryotes, transcription termination and RNA processing are required for mRNA 3' end production (Altman and Robertson, 1973; Zhao et al., 1999; Nudler and Gottesman, 2002). As a result, understanding the mechanisms for producing transcription termination requires an examination of the 3' end of an mRNA regarding its position and relative amount.
The 3’ end of a specific transcript could be identified and located using the 3’ random amplification of cDNA ends (RACE) assay (Sambrook and Russell, 2006; Wang, X. et al., 2018). This assay is based on the ligation of an RNA oligomer to the 3’ end of an RNA transcript, which is amplified using RT-PCR (Lee et al., 2008). Using gene-specific primer extension (PE) and DNA sequencing gel electrophoresis, the 3' end position is determined (Lee et al., 2008). The labeled primer embedded in PCR products is extended by a denaturant PAGE analysis, enabling the visualization and quantification of the 3' end. Using the 3’ RACE-PE method, the gal operon mRNA 3′ end has been previously identified in vivo and studied (Lee et al., 2008; Wang, X. et al., 2014, 2015, 2019; Jeon et al., 2021). The Escherichia coli gal operon has four structural genes, galE, galT, galK, and galM, which encode enzymes for catabolic and anabolic metabolism of D-galactose (Holden et al., 2003). Transcription initiated from the P1 and P2 promoters (Adhya and Miller, 1979) terminate at the terminator hairpin of the last structural gene galM (Wang, X. et al., 2019), generating the 3’ end of galETKM mRNA at 4,313 (from the transcription initiation site of the P1 promoter, +1).
In E. coli, transcription is tightly coupled to translation (Burmann et al., 2010; Chakrabarti and Gorini, 1975; Proshkin et al., 2010), mediating the translation of the nascent transcript (Demo et al., 2017; Fan et al., 2017; Kohler et al., 2017; Wang, C. et al., 2020; Webster et al., 2020). The RNA polymerase often transcribes open reading frames (ORFs) without a linked ribosome, resulting in ribosome-free mRNA (Chen and Fredrick, 2018; Zhu et al., 2019; Jeon et al., 2020). To understand the mechanism of transcription termination for the above-mentioned transcription states, we need specifically targeted tools and methodology. Cell-free expression or in vitro transcription-translation techniques can be utilized for studying functional protein synthesis without requiring a living cell (Silverman et al., 2020). The coupled transcription-translation system provides a time-saving alternative to conventional methods, by coupling both processes in a microcentrifuge tube (Figure 1). Unlike other in vitro approaches based on cell culture, the in vitro transcription-translation system does not require gene transfection, bacterial cell culture, or extensive purification (Silverman et al., 2019, 2020). Although various cell-free extracts have recently been created, E. coli extracts remain the most practical and best choice for protein production due to their high yields (Silverman et al., 2020). For the first time, we report the extraction of RNA from a cell-free system to investigate the mechanism of 3’ end generation in an in vitro system, where transcription is coupled with translation. The in vitro transcription-translation system is comprised of two basic components: (1) the template DNA containing RNA polymerase (RNAP) promoters, and (2) a reaction buffer supplemented with the necessary components for the transcription and translation processes (Kim et al., 2007).
Cell extracts contain all the essential molecules such as RNAP, ribosomes, tRNAs, amino acids, several other cofactors, and a source of energy for transcription and translation (Kim et al., 2007). Any template DNA with an E. coli holoenzyme or T7 RNA polymerase promoter, ribosome binding site (rbs), and terminator (Takahashi et al., 2015) can be used to study protein-protein, protein-nucleic acid interactions (Wong and Blobel, 2008; Tando et al., 2010; Muratore et al., 2012), or transcription termination (Ray-Soni et al., 2016). For instance, to examine Rho-dependent termination, cloning the transcription terminator rho gene in a template with the T7 promoter/terminator and an rbs can be employed. Although in vitro expression is impractical for extensive commercial production, it can support RNA/protein synthesis as a coupled reaction (transcription and translation), making it significantly useful and manageable for many research applications (Tinafar et al., 2019; Silverman et al., 2020).
So far, numerous methodologies have been described for cell-free protein expression (Cole et al., 2020). Here, we present an optimized protocol that combines a cell-free extract system and a 3’ RACE assay, to better understand mRNA generating mechanisms (Jeon et al., 2021). We investigated how transcription termination could be studied in a naturally occurring dual terminator system influenced by transcription-translation coupling. We also show how RNA extracted from a cell-free reaction contains pre-mRNAs previously unseen before processing and after RDT. We anticipate that our in vitro-based experimental approach will be relevant in future studies of gene regulation.
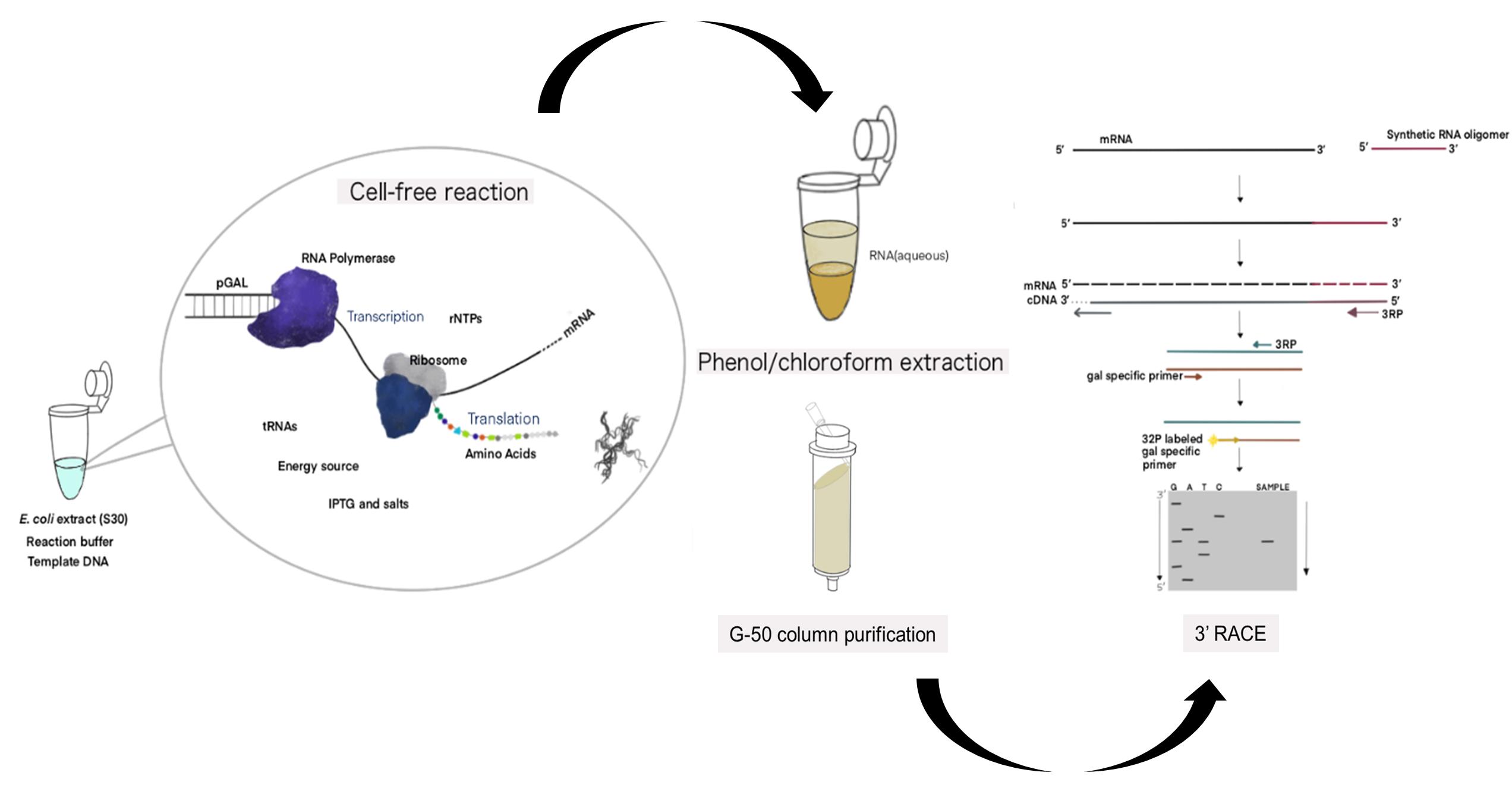
Figure 1. Schematic of the cell-free expression system (CFS) followed by the 3' RACE-PE (CFS-3’RACE-PE) assay. Target RNA is obtained from the template DNA (pGal) using the E. coli extract containing RNA polymerase, ribosome, rNTPs, tRNAs, amino acids, energy source, other components supplied, and a reaction buffer via a coupled transcription-translation reaction, followed by phenol-chloroform extraction and G-50 column purification to purify RNA for use in the RACE assay.
Materials and Reagents
Materials
Pipette (STARLAB International, catalog numbers: S7100-0510, S7100-2200, and S7110-1000)
Pipette tips (Starlabs., S Korea and Sorenson Bioscience, Inc., catalog numbers: S1120-3810, S1122-1830, 2937T, 17370-X)
1.5 mL microcentrifuge tubes (SPL Life Sciences, catalog number: SPL-060015)
1.5 mL microcentrifuge tubes, sterilized (Bio-Fact, catalog number: EMT-1530)
Plasmid DNA mini-prep kit (Bio-Medic, catalog number: BM-K110B)
PCR tubes (Corning Incorporated, catalog number: PCR-02-C)
PCR purification kit (Qiagen, catalog number: 28106)
Whatman 3MM paper (GE Healthcare, catalog number: 3017-915)
Kodak CL-Xposure Film (Thermo Scientific, catalog number: 34090)
Micro-flex Gloves (Ansell, catalog number: MF-300-L)
RNaseZap (RNase Decontamination Solution) (Thermo Scientific, catalog number: AM9780)
E. coli extract (S30) (Bioneer, catalog number: K-7250)
Reaction buffer for cell-free reaction (Bioneer, catalog number: K-7250)
DEPC water (Bioneer, catalog number: K-7250)
RNase-free water (Thermo Scientific, catalog number: 7732-18-5)
Galactose (Merck, catalog number: G5388)
DNase I (Thermo Fisher Scientific, Invitrogen, catalog number: AM2222)
T4 RNA ligase (Ambion, catalog number: AM2141)
RNasin Ribonuclease Inhibitors (Promega, catalog number: N2111)
PCI (Phenol:Chloroform:Isoamyl Alcohol) (Merck, catalog number: 77617)
G-50 column (Sephadex) (GE Healthcare, catalog number: 27-5330-01)
Omniscript Reverse Transcriptase (Qiagen, catalog number: 205111)
HotStarTaq Plus DNA Polymerase (Qiagen, catalog number: 203603)
dNTP mix (10 mM each) (ThermoFisher, catalog number: R0191)
T4 Polynucleotide Kinase (NEB, catalog number: M0201L)
T4 Polynucleotide Kinase 10× buffer (NEB, catalog number: M0201L)
ATP, [γ-32P]-6000Ci/mmol (PerkinElmer, catalog number: NEG002Z250UC)
5× TBE (Bioneer, catalog number: C-9002)
Urea (Merck, catalog number: U5128)
Acrylamide (Merck, catalog number: V900845)
Bis-acrylamide (Merck, catalog number: V900301)
Ammonium persulfate (Merck, catalog number: 248614)
TEMED (Merck, catalog number: T9281)
Formamide (Merck, catalog number: F9037)
Xylene cyanol (Sigma, catalog number: X4126)
Bromphenol blue (Sigma, catalog number: B0126)
Sigmacote (Sigma, catalog number: SL2)
5× Developer (Vivid, catalog number: 0514_00004)
5× Developer & Fixer (Vivid, catalog number: 0514_00003)
8% sequencing solution (see Recipes)
8% Urea-PAGE gel (see Recipes)
2× stop buffer (see Recipes)
Plasmids and primers (Table 1)
pGal plasmid (contains gal operon, Figure 3A) (Wang, X. et al., 2014)
pRho plasmid (contains rho gene under the T7 promotor control) (Jeon et al., 2021)
pRNaseIII (contains rnc gene under the T7 promoter control) (Jeon et al., 2021)
Table 1. Primers used in this study.
Primer name Primer sequence (5’-3’) Use Synthetic RNA UUCACUGUUCUUAGCGGCCGCAUGCUC RNA Oligomer ligation for 3′ RACE assay 3RP-R AGCATGCGGCCGCTAAGAAC RT and PCR for 3′ RACE assay M3-F TCCGCACGACGGCCTGAAAT
PCR for 3′ RACE assayT3-F ACGGTAGCCGTACCGTTGTC M4240-R CGAAGAGTATTCCAGCCTG
PE for 3’ RACE assayT8-F ATCCATTTTCGCGAATCCGGA
Equipment
30/37°C stationary incubator (SNT, model: MCL-20A)
Vortex Genie-2 (Scientific Industries, model: G560E)
37°C Heat block (FINEPCR, model: ALB64)
Centrifuge (4°C) (Hanil Scientific Inc., model: SU-R17)
NanoDrop 1000 (Thermo Fisher, model: ND-1000)
Thermo cycler machine (Bio-Rad, model: T100, catalog number: 1861096)
Gel sequencing apparatus (Labrepco, model: S2, catalog number: 21105036)
Automatic pipette (Thermo Scientific, USA, model: S1 pipette filler, catalog number: 9501)
Cassette (X-ray film) (Duksan (DS) Lab, model: L-07019-001)
Power supply unit (Bio-Rad, USA, model: PowerPac 3000, catalog number: 165-5057)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
N, M. P. A. and Lim, H. M. (2022). An in vitro Assay of mRNA 3’ end Using the E. coli Cell-free Expression System. Bio-protocol 12(4): e4333. DOI: 10.21769/BioProtoc.4333.
分类
微生物学 > 微生物遗传学 > RNA > 转录
生物工程 > 合成生物学
分子生物学 > RNA > miRNA 干扰
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link