- EN - English
- CN - 中文
Femtoliter Injection of ESCRT-III Proteins into Adhered Giant Unilamellar Vesicles
将Escrt-Ⅲ蛋白注射到粘附的巨大单层囊泡中
(*contributed equally to this work) 发布: 2022年02月20日第12卷第4期 DOI: 10.21769/BioProtoc.4328 浏览次数: 3714
评审: Anonymous reviewer(s)
Abstract
The endosomal sorting complex required for transport (ESCRT) machinery mediates membrane fission reactions that exhibit a different topology from that observed in clathrin-coated vesicles. In all of the ESCRT-mediated events, the nascent vesicle buds away from the cytosol. However, ESCRT proteins are able to act upon membranes with different geometries. For instance, the formation of multivesicular bodies (MVBs) and the biogenesis of extracellular vesicles both require the participation of the ESCRT-III sub-complex, and they differ in their initial membrane geometry before budding starts: the protein complex acts either from outside the membrane organelle (causing inward budding) or from within (causing outward budding). Several studies have reconstituted the action of the ESCRT-III subunits in supported bilayers and cell-sized vesicles mimicking the geometry occurring during MVBs formation (in-bud), but extracellular vesicle budding (out-bud) mechanisms remain less explored, because of the outstanding difficulties encountered in encapsulation of functional ESCRT-III in vesicles. Here, we provide a different approach that allows the recreation of the out-bud formation, by combining giant unilamellar vesicles as a membrane model and a microinjection system. The vesicles are immobilized prior to injection via weak adhesion to the chamber coverslip, which also ensures preserving the membrane excess area required for budding. After protein injection, vesicles exhibit outward budding. The approach presented in this work can be used in the future to disentangle the mechanisms underlying ESCRT-III-mediated fission, recreating the geometry of extracellular bud production, which remains a challenge. Moreover, the microinjection methodology can be also adapted to interrogate the action of other cytosolic components on the encapsulating membranous organelle.
Graphic abstract:
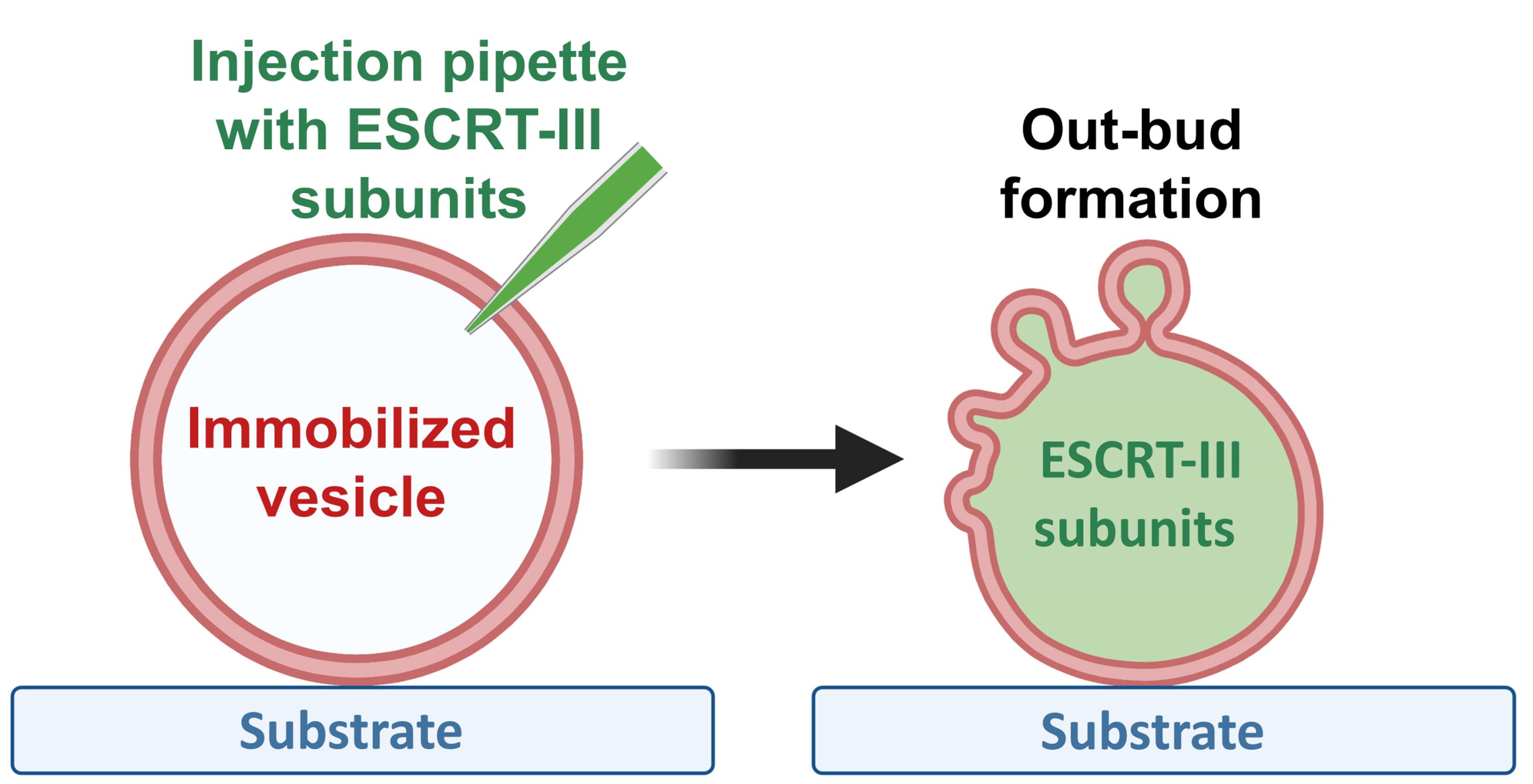
Out-bud formation after ESCRT-III protein injection into GUVs
Background
Extracellular vesicles (EVs) are defined as cell-derived vesicles confined by a lipid bilayer. They participate in cellular disposal and in intercellular communication by delivering antigens, genetic material, and lipids to recipient cells (Raposo and Stahl, 2019). EV secretion seems to be an ubiquitous process present throughout all domains of life and, in most of the cases, it is related to normal physiological conditions (Herrmann et al., 2021). However, a considerable increase in EV number is observed during pathogenic processes including cancer (Wolfers et al., 2001; Tao et al., 2019; Clos-Garcia et al., 2018; Xu et al., 2018), hereditary α-tryptasemia (Glover et al., 2019), multiple sclerosis (Moyano et al., 2016), cytotoxic drug intoxication (Keklikoglou et al., 2019), and parasitic diseases, among which cerebral malaria has been widely characterized (Combes et al., 2004; Schofield and Grau, 2005; Campos et al., 2010; Mfonkeu et al., 2010; Nantakomol et al., 2011; Martin-Jaular et al., 2011). EVs can be classified into two major classes, depending on their size and origin: exosomes, with a typical diameter of 30–150 nm, and microvesicles, that have a diameter of 100–1,000 nm (Raposo and Stahl, 2019). Whereas microvesicles are shed by outward budding of the plasma membrane, exosomes are generated by the fusion of multivesicular bodies (MVBs) with the plasma membrane, followed by the release of intraluminal vesicles (ILVs) (van Niel et al., 2018). EV populations are considered highly heterogeneous both in content and in size, probably due to the different pathways along which they originate from (Raposo and Stahl, 2019), making it difficult to resolve the mechanisms associated with cargo-packing and specific activity during cell signaling. Understanding the molecular processes that govern their biogenesis, via employing simple mimetic systems, could provide a clue to solve their mechanism of action, and therefore, help to understand the pathophysiology of certain diseases.
One of the pathways for EV biogenesis is orchestrated by the endosomal sorting complex required for transport (ESCRT) machinery, which comprises several protein subunits organized into four different complexes (ESCRT-0, -I, -II and -III), and the accessory Vps4 complex (reviewed in Vietri et al., 2020). Typically, ESCRT members are recruited to the endosomal membrane in a stepwise manner, with subsequent activation of the different ESCRT complexes. Two non-canonical pathways have been also identified: 1) activation of ESCRT-I by Bro1, which functions as an ubiquitin acceptor (Tang et al., 2016), and 2) ESCRT-III activation by Alix, which mediates the ubiquitin-independent, but ESCRT-III-dependent endocytosis (Dores et al., 2012). On the other hand, the ESCRT machinery has also been implicated in the production of nano-sized vesicles that are enriched in cell surface proteins, reflecting its participation during microvesicle formation (Nabhan et al., 2012; Wang and Lu, 2017).
ESCRT-III recruitment in Plasmodium falciparum operates through a non-canonical pathway in which PfBro1 activates PfVps32 and PfVps60, both ESCRT-III members, triggering EV biogenesis (Avalos-Padilla et al., 2021b). Involving an intracellular parasite, the study of this process is problematic. Moreover, the knockdown or deletion of ESCRT genes in other organisms results in the formation of aberrant structures that lack ILVs (Doyotte et al., 2005; Nickerson et al., 2006). To address these difficulties, membrane models have been widely used to analyze in vitro ESCRT-III-mediated events. In this direction, giant unilamellar vesicles (GUVs) (Dimova and Marques, 2019; Dimova, 2019) combined with recombinant ESCRT proteins have become an established platform to examine the formation of MVBs (Im et al., 2009; Avalos-Padilla et al., 2018 and 2021a; Booth et al., 2019;Alqabandi et al., 2021). To mimic the geometry occurring in this process, ESCRT components are introduced in the vesicle surroundings. The proteins induce membrane invaginations towards the vesicle interior, which can lead to the formation of ILVs, connected to the mother membrane through a thin neck, and the final cleavage of the neck results in the formation of MVB-like GUVs.
The out-budding processes (as in the formation of microvesicles shed by the plasma membrane) exhibit a reverse budding topology, compared to that of MVB formation. Thus, to explore such processes, the ESCRT units have to act from within the vesicle model. In other words, the proteins have to be introduced into the GUVs’ lumen. One approach to accomplish this consists of encapsulating ESCRT proteins inside GUVs, by forming the vesicles in the presence of the proteins; using this strategy, nanotubes with the correct topology for scission were pulled and subsequently cleaved (Schöneberg et al., 2018). However, under these conditions, one cannot observe the vesicle response upon immediate interaction with the proteins, as it relies on ATP photo-uncaging. To evade these drawbacks, we have designed an approach in which pre-formed GUVs encapsulating the buffer necessary for protein activity are injected with the ESCRT-III proteins. With this technique, we are able to observe in real time the dynamics of out-bud formation (mimicking the process driven during EV biogenesis), and to evaluate the effects specific to a particular protein.
Injection approaches in GUVs have been applied previously (Wick et al., 1996; Hurtig and Orwar, 2008; Lefrançois et al., 2018). In isolated GUVs, it is important to ensure control over the vesicle volume and area. In particular, the injection of isotonic solutions can pull out the excess membrane area needed for deformation, which would then prohibit outward budding. Thus, we adapted this protocol, by employing osmolarities of the injected solutions which lead to vesicle deflation, to create excess vesicle area for deformation and budding. Furthermore, isolated vesicles need to be immobilized to facilitate puncturing the membrane without displacing and losing them. In previous work, the immobilization was ensured by working with GUVs which were directly formed on the substrate for GUV swelling. However, such vesicles are typically connected to other GUVs and structures, thus not ensuring area/volume conservation. Here, we employed biotin-avidin-based adhesion, using biotinylated lipids in the vesicle and an avidin-coated substrate to which the GUVs were fixed as proposed earlier (Maan et al., 2018). Successful injection of ESCRTs and further registration of the functionality of the proteins, namely formation of out-buds, requires fine adjustment of the adhesion level. On one hand, strong adhesion stabilizes the vesicles during the puncturing procedure, while on the other hand, it increases the membrane tension while consuming the area available for deformation. The latter effect limits the ability of the membrane to bend and thus hinders budding. We thus optimized the avidin surface concentration to ensure mild adhesion. Another important aspect to consider is the buffer in which proteins remain active: in the case of ESCRT-III proteins, the buffer used was 150 mM NaCl and 25 mM Tris-HCl, pH 7.4 (~325 mOsmol/kg). This high salt concentration hamperedGUVs growing through the standard electroformation protocol (Angelova and Dimitrov, 1986). Despite some recent developments of this protocol aiming at application to solutions of high ionic strength (Montes et al., 2007; Pott et al., 2008; Li et al., 2016; Lefrançois et al., 2018), it is clear that the incorporation of negatively charged lipids imposes strong limitations, resulting in poor vesicle quality and small size (as confirmed by our own tests, data not shown). To overcome these drawbacks, we used the gel-assisted method (Weinberger et al., 2013), in which we were able to grow vesicles encapsulating the protein buffer. It must be noted that this method may lead to the incorporation of polymers into the formed vesicles, thus altering their mechanical properties (Dao et al., 2017). Indeed, occasionally we observed vesicles with denser content (presumably resulting from encapsulated hydrogel). For the work here, we always selected “clean” GUVs with no visible alterations in their membranes and, as detailed later, substantiate the results with control experiments where vesicles were injected with protein-free buffer. As we are working with P. falciparum ESCRT-III subunits, the GUV lipid composition was selected to mimic the inner leaflet of the red blood cell plasma membrane (Virtanen et al., 1998). However, this can be modified depending on the system. We also demonstrate the injection and outward budding process for GUVs injected with another ESCRT-III system, namely the protozoan parasite responsible for amoebiasis, Entamoeba histolytica, whose characterization in GUVs has been previously reported (Avalos-Padilla et al., 2018), using a suitable membrane composition. Finally, as mentioned above, to deflate the GUVs, and thus to ensure that the vesicles exhibit excess membrane area needed for the formation of the out-buds, the injected proteins were kept in a 0.8× buffer (prepared by a direct dilution in Milli-Q water of the 1× protein buffer, see Recipe 4). As a control, upon injection of GUVs with the same volume and osmolarity of protein-free solution as in the experimental set-up, no out-buds appeared, demonstrating the validity of our approach.
Materials and Reagents
Lipids
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine [POPC] (Avanti Polar Lipids, catalog number: 850457)
1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine [POPS] (Avanti Polar Lipids, catalog number: 840034)
Cholesterol (ovine) [chol] (Avanti Polar Lipids, catalog number: 700000)
1,2-dioleoyl-sn-glycero-3-phospho-(1’-myo-inositol-3’-phosphate) [PI(3)P] (Avanti Polar Lipids, catalog number: 850150)
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[biotinyl(polyethyleneglycol)-2000] [DSPE-PEG 2000 Biotin] (Avanti Polar Lipids, catalog number: 880129)
1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) [DPPE-Rhodamine] (Avanti Polar Lipids, catalog number: 810158)
Note: All lipids are dissolved in chloroform at a final concentration of 10 mg·mL-1 and can be stored at -20°C for up to one month.
Reagents
Avidin from egg white (Sigma-Aldrich, catalog number: A9275)
Bovine serum albumin [BSA] (Sigma-Aldrich, catalog number: A8806)
Biotinylated BSA (Thermo Fisher Scientific, catalog number: 29130)
Chloroform (Merck, Supelco, catalog number: 288306)
Ethanol absolute (Merck, catalog number: 1009831011)
Methanol (Merck, catalog number: 34860)
Milli-Q® water (Millipore system)
Polyvinyl alcohol [PVA] (Approximate MW 145,000 g·mol-1, Merck, catalog number: 814894)
Polyethylene glycol fluorescein isothiocyanate [PEG-FITC] (Nanocs, catalog number: PG1-FC-40k)
NaCl (Sigma-Aldrich, catalog number: S7653)
Trizma® hydrochloride (Sigma-Aldrich, catalog number: T6666)
Purified recombinant PfBro1 and PfVps32 from P. falciparum (Avalos-Padilla et al., 2021b)
Purified recombinant EhVps20t and EhVps32 from E. histolytica (Avalos-Padilla et al., 2018)
PVA solution (5% w/v) (see Recipe 1)
Lipid mix (1 mg·mL-1) for the injection of recombinant P. falciparum PfBro1 and PfVps32 in vesicles made of POPC:POPS:DSPE-PEG 2000 Biotin:DPPE-Rhodamine (see Recipe 2)
Lipid mix (1 mg·mL-1) for the injection of recombinant E. histolytica EhVps20t and EhVps32 in vesicles made of POPC:POPS:chol:PI(3)P:DSPE-PEG 2000 Biotin:DPPE-Rhodamine (see Recipe 3)
Protein buffer 1×, pH 7.4 (see Recipe 4)
Note: The purification of the P. falciparum proteins (PfBro1 and PfVps32) and E. histolytica proteins (EhVps20t and EhVps32) followed a conventional protocol of affinity purification using the GST-tag present in the recombinant proteins and GSH-Sepharose 4B for its capture. The GST-tag was later removed, and recombinant proteins were purified by size exclusion chromatography as detailed in Avalos-Padilla et al. (2021b) and Avalos-Padilla et al. (2018), respectively.
Consumables
Thin wall borosilicate capillaries with filament (internal radius: 0.78 mm, external radius: 1 mm), to be used in the fabrication of the injection micropipettes (Harvard Apparatus, catalog number: 30-0038)
Cover glass (Menzel Gläser, 26 mm × 56 mm, 0.17 mm thick, custom made)
Hamilton syringe 1 mL (Carl Roth, catalog number: EY44.1)
0.22 µm membrane filter (Milex-GV, catalog number: SLGVR04NL)
Silicone paste (Korasilon-Paste, Carl Roth, catalog number: 0857.1)
2 mm thick homemade Teflon spacer (length/width 40/22 mm), see Figure 2
Eppendorf® microloader 20 µL tips (Eppendorf, catalog number: 5242956003)
Glass vial for the lipid stock solution (DKW Life Science, catalog number: 11768929)
Glass vial for the PVA solution (rollrandglaeser; Carl Roth, catalog number: X661.1)
Pipette tips (capacity 1,000 µL; Merck, catalog number: AXYT1000B)
Weighing pan ROTILABO® (Carl Roth, catalog number: 2149.2)
Measuring vessels for Osmomat 3000 (Gonotec, catalog number: 30.9.0010)
Equipment
Oven (Thermo Fisher Scientific, Heraeus Vacutherm)
Vacuum pump (Vacuubrand, model: MZ 2C NT + 2AK)
Micropipette puller (Sutter Instruments, model: P-97)
Micropipette manipulator system (Sutter Instruments, model: MPC-385)
Microinjector (Eppendorf, model: FemtoJet 5247)
Confocal microscope (Leica TCS SP5 equipped with an oil-immersion objective, 63×, NA 1.4)
Vacuum desiccator ROTILABO® (Carl Roth, article No. 1008.1)
Milli-Q® system (SG water purification system, Integra UV plus, 18.2 MΩ·cm)
Variable volume pipette (0.12 µL, 0.510 µL, 1001,000 µL, Eppendorf®)
Magnetic stirrer (IKA Werke Staufen, type: Bigsquid)
Magnetic stirrer bars (Fisherbrand, catalog number: 11834792)
Osmometer (Gonotec, Osmomat 3000 freezing point osmometer)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Georgiev, V. N., Avalos-Padilla, Y., Fernàndez-Busquets, X. and Dimova, R. (2022). Femtoliter Injection of ESCRT-III Proteins into Adhered Giant Unilamellar Vesicles. Bio-protocol 12(4): e4328. DOI: 10.21769/BioProtoc.4328.
分类
生物物理学 > 显微技术
生物工程 > 合成生物学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













