- EN - English
- CN - 中文
A Hypersensitive Genetically Encoded Fluorescent Indicator (roGFP2-Prx1) Enables Continuous Measurement of Intracellular H2O2 during Cell Micro-cultivation
超灵敏基因编码荧光指示剂 (roGFP2-Prx1) 在细胞微培养期间连续测量细胞内 H2O2
发布: 2022年02月05日第12卷第3期 DOI: 10.21769/BioProtoc.4317 浏览次数: 3333
评审: Alexandros AlexandratosGonzalo Durante-RodríguezKomuraiah MyakalaAnonymous reviewer(s)

相关实验方案
用于比较人冷冻保存 PBMC 与全血中 JAK/STAT 信号通路的双磷酸化 CyTOF 流程
Ilyssa E. Ramos [...] James M. Cherry
2025年11月20日 2283 阅读
Abstract
Hydrogen peroxide (H2O2) is a toxic oxidant produced as a byproduct of several biological processes. At too high levels of hydrogen peroxide cells will experience oxidative stress, leading to a cellular response to decrease its levels and to protect the cells. Previously, methods used to study and quantify intracellular H2O2 have been limited by both sensitivity and specificity. However, an increasing number of genetically encoded fluorescent indicators (GEFIs) are becoming available, which can specifically detect low levels of intracellular hydrogen peroxide. In this study, we use such a biosensor designed to monitor cytosolic H2O2 levels in the budding yeast Saccharomyces cerevisiae during continuous cultivation and in the absence of a fluorescence microscope. The fluorescent biosensor contains a peroxiredoxin protein fused to an engineered GFP molecule expressed from a commonly used yeast plasmid (pRS416-TEF1). The peroxiredoxin-based fluorescent indicator reduces H2O2, ultimately resulting in a GFP signal being emitted by the sensor. Here, we apply this biosensor to study cytosolic H2O2 levels in S. cerevisiae strains with and without recombinant protein production.
Graphic abstract:
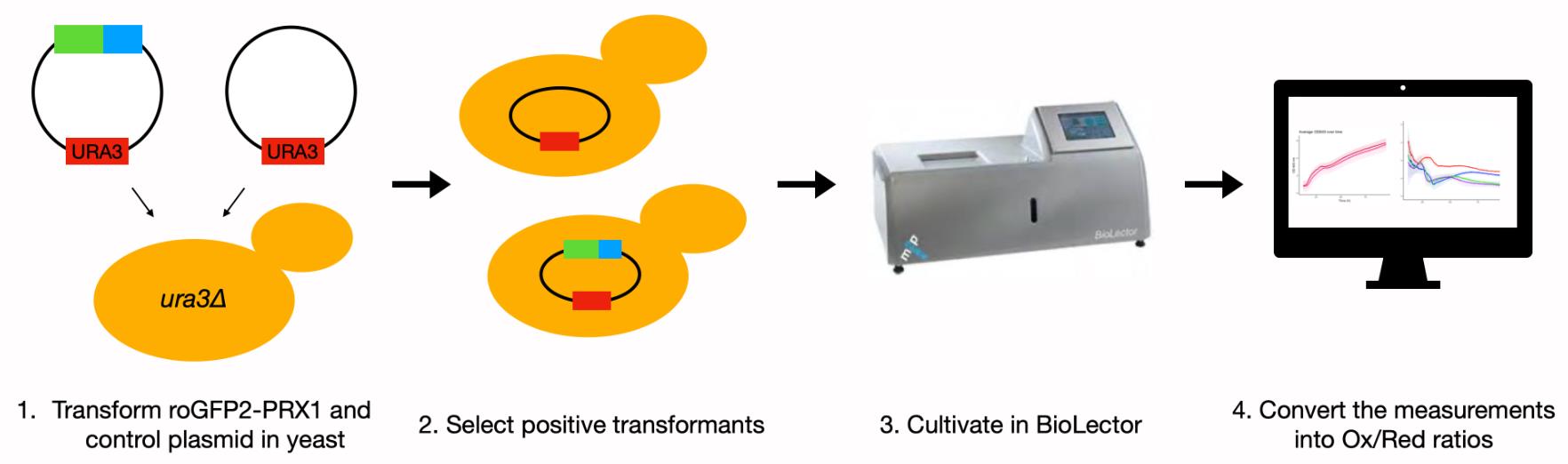
Schematic overview of experimental steps.
Background
In this protocol, we discuss the use of a hypersensitive hydrogen peroxide (H2O2) sensor, roGFP2-Prx1, in the yeast Saccharomyces cerevisiae. S. cerevisiae is a broadly used industrial yeast species but also a popular model organism to study eukaryotic cellular biology. Within biological systems, this includes the generation and buildup of unwanted toxins like H2O2. When oxygen is incompletely reduced to water, reactive oxygen species such as H2O2 are produced. If the intracellular level of H2O2 becomes too high, the cell will endure oxidative stress, which drains resources and increases maintenance costs (Jamieson, 1998; Malhotra et al., 2008; Dever et al., 2016). Oxidative phosphorylation in the mitochondria is responsible for a major part of the H2O2 generated in eukaryotic cells (Malhotra et al., 2008). However, another important source of H2O2 is the production of proteins destined for secretion, and more specifically their folding. The H2O2 resulting from secretory protein production is formed in the iterative process of the making and breaking of disulfide bonds before the proteins reach their final folded state. Especially for recombinant protein production, it is often the goal to achieve high protein titers and yields, which means a high flow through the secretory pathway and the folding machinery in the endoplasmic reticulum (ER). Increased secretory protein folding will lead to an elevated production of H2O2, and therefore potentially oxidative stress.
For many years, methods for measurement of intracellular reactive oxygen species (ROS), including H2O2, consisted of different ROS-sensitive dyes. These dyes are known for their undefined specificity and poor resolution, both temporally and spatially (Morgan et al., 2016). A new technology circumventing these problems was introduced in the form of genetically encoded fluorescent indicators (GEFIs) (Lukyanov and Belousov, 2014). GEFIs are fluorescent biosensors that enable real-time monitoring of several cellular processes but also of relevant compounds like H2O2 (Lukyanov and Belousov, 2014). The basis for the specific GEFI used in this protocol is a fusion of two proteins, which form a redox pair when together. One of these is a highly H2O2-reactive peroxiredoxin e.g., yeast Prx1, and the other a green fluorescent protein, roGFP2. RoGFP2 is an engineered enhanced GFP that has two cysteines in close proximity to the chromophore. Upon the making or breaking of a disulfide bond between those two cysteines, there will be a change in the protonation state of the chromophore, which changes the excitation wavelength (Schwarzländer et al., 2016). With the reduction of H2O2, the two cysteines in Prx1 form a disulfide bond that will next react into a mixed disulfide bond between Prx1 and the roGFP2 molecule; this finally resolves into an internal disulfide bond within roGFP2, thereby changing the excitation wavelength. A schematic overview of this mechanism is shown in Figure 1. RoGFP2 has excitation peaks at 488 nm in the reduced form and at 400 nm in the oxidized form that carries the internal disulfide bond (Schwarzländer et al., 2016). By dividing the signal measured at 400 nm by the signal measured at 488 nm, an oxidized:reduced ratio can be determined. At a basal level of endogenous H2O2 under non-stressed conditions, the sensor maintains an oxidized:reduced ratio near 1:1 (Schwarzländer et al., 2016). Furthermore, the sensor is ratiometric, and therefore any potential differences in sensor abundance between strains and/or conditions are expected not to affect measurements. The functionality and sensitivity of these sensors in cells have been tested by the direct addition of exogenous H2O2, but also a reducing agent (DTT), to the media (Morgan et al., 2016; Gast et al., 2021). Importantly, the roGFP2-Prx1 sensor can detect both oxidizing and reducing changes in the extracellular environment.
Here, we present a protocol on the use of the roGFP2-Prx1 sensor for in vivo measurements of cytosolic H2O2 during continuous micro-cultivation of S. cerevisiae in a BioLector (Mp2 Labs). This protocol was successfully used to detect changes in cytosolic H2O2 levels in S. cerevisiae due to recombinant protein production (Gast et al., 2021). The sensors are expected to be adequate to measure H2O2 produced from sources other than recombinant protein production as well.
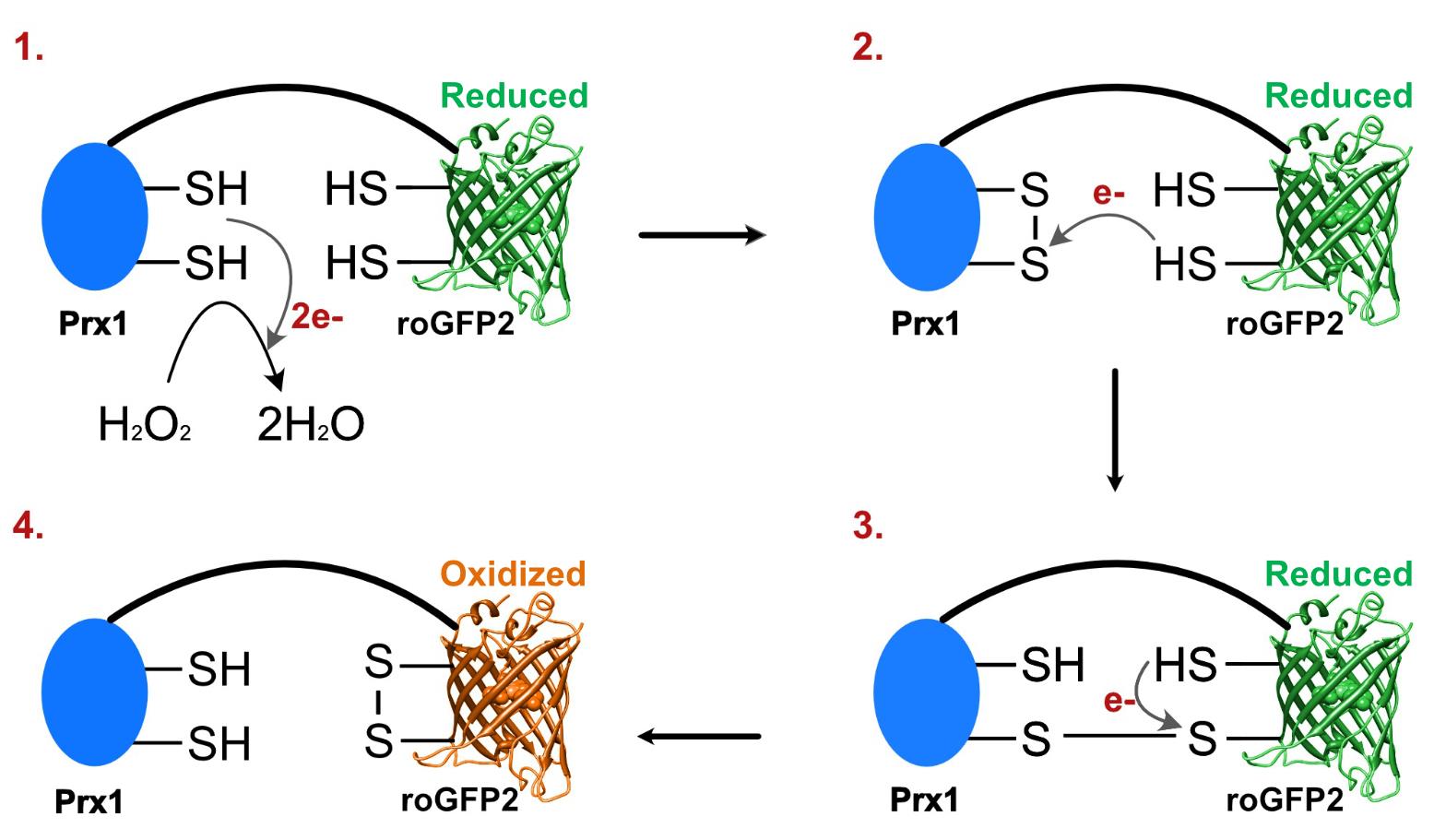
Figure 1. H2O2 oxidizes cysteines in Prx1-roGFP2 into a disulfide bond altering sensor fluorescence. (1) Prx1 in the genetically-encoded fluorescent indicator reduces H2O2 to water by making an internal disulfide bond. (2) One of the electrons of a cysteine in the roGFP2 reacts with one of the cysteines in the disulfide bond, resulting in a new disulfide bond between Prx1 and roGFP2. (3) The second cysteine in roGFP2 reacts with the other cysteine in the disulfide bond between Prx1 and roGFP2, resulting in a new disulfide bond between the two cysteines in roGFP2. (4) The disulfide bond between the cysteines in roGFP2 changes the equilibrium of different forms of the chromophore, resulting in a shift of the wavelength of maximal excitation of roGFP2 from 488 to 400 nm.
Materials and Reagents
Materials
48-well flowerplates (Mp2 Labs, catalog number: MTP-48-B)
Sealing foil reduced evaporation (Mp2 Labs, catalog number: F-GPR48-10)
Sterile 1.5 mL tubes (Eppendorf, catalog number: 0030120086)
Culture tubes (VWR, catalog number: 211-0082)
Cuvettes PS (VWR, catalog number: 634-0676)
50 mL Falcon tubes (Corning, catalog number: 352070)
Petri dishes (Sigma-Aldrich, catalog number: P5731)
Biological materials and reagents
S. cerevisiae strain B184 ura3Δ
Plasmid pRS416-TEF1-roGFP2-PRX1 (Morgan et al., 2016)
Plasmid pRS416M1-TEF1 (control) (Kaur and Bachhawat, 2009), but plasmid pRS416-TEF1 works equally well. pRS416M1-TEF1 was constructed by removing all the restriction sites in the cloning site of pRS416-TEF1, except for BamHI and EcoRI.
Boiled ss-carrier DNA
Sterile H2O (store at RT)
Sterile 0.1 M LiAc (store at RT)
YNB w/o AA (Formedium, catalog number: CYN0405)
NaH2PO4·2H2O (Merck, catalog number: 06342)
Na2HPO4 (Merck, catalog number: 06586)
Glucose·H2O (Merck, catalog number: 08342)
BSA (Sigma-Aldrich, catalog number: A7030)
Arginine (Sigma-Aldrich, catalog number: A5006)
Aspartic acid (Sigma-Aldrich, catalog number: A9256)
Glutamic acid (Sigma-Aldrich, catalog number: G1251)
Glycine (Sigma-Aldrich, catalog number: G7126)
Histidine (Sigma-Aldrich, catalog number: H8000)
Isoleucine (Sigma-Aldrich, catalog number: I2752)
Leucine (Sigma-Aldrich, catalog number: L8000)
Methionine (Sigma-Aldrich, catalog number: M9625)
Phenylalanine (Sigma-Aldrich, catalog number: P2126)
Threonine (Sigma-Aldrich, catalog number: T8625)
Tryptophan (Sigma-Aldrich, catalog number: T0254)
Tyrosine (Sigma-Aldrich, catalog number: T3754)
Valine (Sigma-Aldrich, catalog number: V0500)
Yeast extract (Fisher Scientific, catalog number: AC451120010)
LiAc (Sigma-Aldrich, catalog number: L6883)
PEG 3350 (Sigma-Aldrich, catalog number: 202444)
Agar (Sigma-Aldrich, catalog number: 05040)
Complete Supplement Mixture Single Drop-Out -Ura (Formedium, catalog number: DCS0169)
SD2xSCAA medium (store at 4°C) (see Recipes)
SD-URA agar plates (store at 4°C) (see Recipes)
Yeast extract peptone dextrose media (YPD) (store at RT) (see Recipes)
Sterile 50% PEG 0.1 M LiAc (store at RT) (see Recipes)
Equipment
Micropipettes (Eppendorf, model: Research® plus)
BioLector I (Mp2 Labs, BioLector)
Emission filters:
a. GFP filter: excitation 480 nm and emission 520 nm (Mp2 Labs, model: E-OP-404)
b. UV-GFP filter: excitation 405 nm and emission 508 nm (Mp2 Labs, model: E-OP-412)
c. Biomass filter: absorption 620 nm (Mp2 Labs, model: E-OP-401)
OD spectrophotometer (ThermoFisher, Genesys 20)
Temperature controlled environment at 30°C
Water bath at 42°C
Shaking platform with 96 culture tubes rack holders (VWR, Advanced orbital Shaker)
Laminar flow hood with UV sterilization
Centrifuge (Eppendorf, model: 5417 R)
Software
RStudio: integrated development environment for R. (2021) (Rstudio Team, Vienna, Austria, https://www.rstudio.com) (optional)
BioLection software (Mp2 Labs)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Gast, V., Siewers, V. and Molin, M. (2022). A Hypersensitive Genetically Encoded Fluorescent Indicator (roGFP2-Prx1) Enables Continuous Measurement of Intracellular H2O2 during Cell Micro-cultivation. Bio-protocol 12(3): e4317. DOI: 10.21769/BioProtoc.4317.
分类
细胞生物学 > 细胞信号传导 > 胁迫反应
微生物学 > 微生物细胞生物学
细胞生物学 > 细胞信号传导 > 胞内信号传导
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link