- EN - English
- CN - 中文
Rapid in vitro and in vivo Evaluation of Antimicrobial Formulations Using Bioluminescent Pathogenic Bacteria
使用生物发光病原菌的抗菌制剂的体内外快速评价
发布: 2022年01月20日第12卷第2期 DOI: 10.21769/BioProtoc.4302 浏览次数: 3915
评审: Aftab NadeemSaumik BasuAnonymous reviewer(s)
Abstract
Basic and translational research needs rapid methods to test antimicrobial formulations. Bioluminescent bacteria and advanced imaging systems capable of acquiring bioluminescence enable us to quickly and longitudinally evaluate the efficacy of antimicrobials. Conventional approaches, such as radial diffusion and viable count assays, are time-consuming and do not allow for longitudinal analysis. Bioluminescence imaging is sensitive and gives vital spatial and temporal information on the infection status in the body. Here, using bioluminescent Pseudomonas aeruginosa, we describe an in vitro and an in vivo approach to rapidly evaluate the antimicrobial efficacy of the host-defense peptide TCP-25.
Graphic abstract:
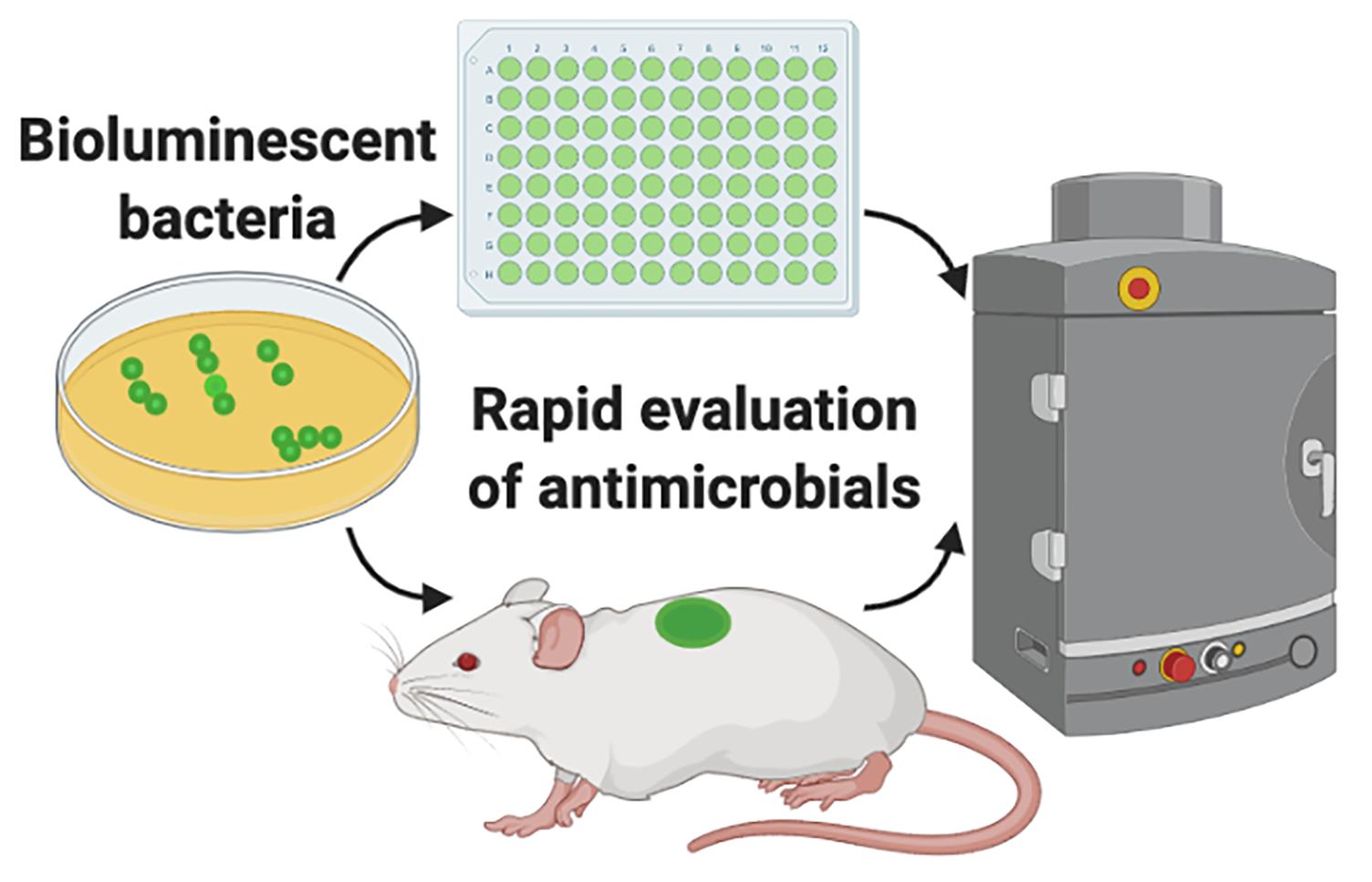
Evaluation of antimicrobials using bioluminescent bacteria.
Background
Both in basic research and in the pharmaceutical industry, there is a high demand for in vitro and in vivo models that can provide quick and reproducible results when testing antimicrobial formulations. Availability of bioluminescent bacteria and advanced imaging systems capable of acquiring bioluminescence originating from in vitro experiments or experimental animal models enable us to quickly and longitudinally evaluate the efficacy of antimicrobials. Conventional in vitro approaches, among many others, include radial diffusion and viable count assays (Balouiri et al., 2016). For in vivo evaluation, CFU (colony-forming unit) enumeration of the animal tissue is the standard practice. Although all these approaches have their own advantages, they are time-consuming as microbiological culturing protocols require 24 to 72 h before results are known. In particular, in vivo evaluation requires that animals be sacrificed for each experimental time point, which renders this process laborious and longitudinal analysis unattainable.
Bioluminescence imaging does not require an external excitation light source. Therefore, for in vivo applications, it is preferred over fluorescence imaging, which often generates a high background, as tissues in the body produce autofluorescence upon excitation with an external source (Choy et al., 2003).
The additional significant advantage is that in vivo bioluminescence imaging gives vital information on spatial distribution of the infection throughout the body (Karimi et al., 2016). Infection development from the original inoculation site can be tracked and its spread can be visualized and quantified. If required, this method can also be combined with fluorescently labeled drugs, so that signals from bioluminescent bacteria and fluorescent drugs can be acquired simultaneously and co-registered, to study their interactions (van Oosten et al., 2013; Puthia et al., 2020). This method can easily be adapted for different antimicrobial products such as gels, aqueous solutions, dressings, and implants. We have successfully used bioluminescent versions of the clinically important pathogenic bacteria Staphylococcus aureus and Pseudomonas aeruginosa for the initial in vitro screening of antimicrobial formulations (Puthia et al., 2020). Employing IVIS imaging, these bioluminescent bacteria were further used by us to evaluate the in vivo efficacy of an antimicrobial formulation in a mouse model of infection using a subcutaneous implant (Strömdahl et al., 2021).
Here, using bioluminescent P. aeruginosa, we describe an in vitro and an in vivo approach to rapidly evaluate the antimicrobial efficacy of the host-defense peptide TCP-25 (thrombin-derived C-terminal peptide). TCP-25 is a thrombin-derived C-terminal peptide (TCP-25, GKYGFYTHVFRLKKWIQKVIDQFGE) that shows strong antimicrobial (Puthia et al., 2020) and antiendotoxic properties (Saravanan et al., 2018). In vivo, TCP-25 protects against P. aeruginosa-induced sepsis and LPS-mediated shock (Papareddy et al., 2010; Kalle et al., 2012). Bioluminescent P. aeruginosa (P. aeruginosa Xen41) is derived from the isolate PAO1 and contains the luxCDABE operon of Photorhabdus luminescens, which is constitutively expressed (Winson et al., 1998; Dusane et al., 2017). Using bioluminescence imaging, we performed longitudinal analysis in both in vitro and in vivo assays, to obtain rapid and valuable results.
Materials and Reagents
Bacterial inoculation loop (Copan, catalog number: 8177CS20H)
Bacterial culture tube, Screw cap, 12 mL (Sarstedt, catalog number: 60.9922.937)
Clear flat bottom 96 well microplate (Greiner Bio-One, catalog number: 655101)
Mepilex® Transfer (Mölnlycke Heath Care, catalog number: 294800)
Biopsy punch, 6 mm (Kai, catalog number: BP-60F)
Petri dish (Sigma-Aldrich, catalog number: P5981)
Alcohol wipes (Cutisoft wipes, BSN Medical, catalog number: 204364)
Gauze swab (SELEFA, ONRMED, Finlan, catalog number: 222002)
BALB/c mice, 8-12 weeks old, male or female (JANVIER LABS, France, BALB/cJRj)
P. aeruginosa Xen41 (PerkinElmer, catalog number: 119229)
BD Bacto Todd Hewitt Broth (Fisher Scientific, catalog number: 249210)
TCP-25 (GKYGFYTHVFRLKKWIQKVIDQFGE)
As an antimicrobial drug, we used the thrombin-derived peptide TCP-25 (synthesized by Biopeptide, San Diego, CA, USA).
VICRYL suture (Johnson & Johnson, Belgium, catalog number: V452H)
Endotoxin free water (Sigma, catalog number: TMS-011-A)
Isoflurane gas (Forane, Baxter, catalog number: CA2L9100)
Tris buffer (10 mM, pH 7.4)
Ketamine (Ketaminol, Intervet, catalog number: 511519)
Xylazine (Rompun vet, BAYER, catalog number: 023572)
Depilatory cream (Veet Hair removal Cream, Reckitt Benckiser, catalog number: 5701092103888)
TCP-25 solution (see Recipes)
TH broth (see Recipes)
Equipment
Surgical scissors (Aesculap, catalog number: BC162R)
Tweezers (Aesculap, catalog number: BD217R)
Shaking incubator (Innova 42, Fisher Scientific, catalog number: 11330025)
In vivo imaging system (IVIS spectrum, PerkinElmer, catalog number: 124262)
Cordless hair clipper (Aesculap, catalog number: GT416)
Spectrophotometer (Genesys 20, Thermo Scientific)
Centrifuge (Sigma 1-6 compact centrifuge, Sigma)
Software
Living Image 4.5.5 Software (PerkinElmer)
Prism, version 8.3.0 (GraphPad Software, LLC.)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Schmidtchen, A. and Puthia, M. (2022). Rapid in vitro and in vivo Evaluation of Antimicrobial Formulations Using Bioluminescent Pathogenic Bacteria . Bio-protocol 12(2): e4302. DOI: 10.21769/BioProtoc.4302.
- Puthia, M., Butrym, M., Petrlova, J., Stromdahl, A. C., Andersson, M. A., Kjellstrom, S. and Schmidtchen, A. (2020). A dual-action peptide-containing hydrogel targets wound infection and inflammation. Sci Transl Med 12(524): eaax6601.
分类
药物发现 > 药物设计
微生物学 > 体内实验模型 > 细菌
生物科学 > 微生物学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













