- EN - English
- CN - 中文
Real-Time Analysis of Mitochondrial Electron Transport Chain Function in Toxoplasma gondii Parasites Using a Seahorse XFe96 Extracellular Flux Analyzer
使用 Seahorse XFe96 细胞外通量分析仪实时分析弓形虫寄生虫的线粒体电子传递链功能
发布: 2022年01月05日第12卷第1期 DOI: 10.21769/BioProtoc.4288 浏览次数: 4173
评审: Alexandros AlexandratosAndrew MacLeanSebastien Besteiro

相关实验方案

TZA, 一种基于敏感报告因子细胞学检测方法, 可用于准确快速量化静息CD4+ T细胞中可诱导的且复制能力强的潜伏HV-1
Anwesha Sanyal [...] Phalguni Gupta
2019年05月20日 7794 阅读
Abstract
The mitochondrial electron transport chain (ETC) performs several critical biological functions, including maintaining mitochondrial membrane potential, serving as an electron sink for important metabolic pathways, and contributing to the generation of ATP via oxidative phosphorylation. The ETC is important for the survival of many eukaryotic organisms, including intracellular parasites such as the apicomplexan Toxoplasma gondii. The ETC of T. gondii and related parasites differs in several ways from the ETC of the mammalian host cells they infect, and can be targeted by anti-parasitic drugs, including the clinically used compound atovaquone. To characterize the function of novel ETC proteins found in the parasite and to identify new ETC inhibitors, a scalable assay that assesses both ETC function and non-mitochondrial parasite metabolism (e.g., glycolysis) is desirable. Here, we describe methods to measure the oxygen consumption rate (OCR) of intact T. gondii parasites and thereby assess ETC function, while simultaneously measuring the extracellular acidification rate (ECAR) as a measure of general parasite metabolism, using a Seahorse XFe96 extracellular flux analyzer. We also describe a method to pinpoint the location of ETC defects and/or the targets of inhibitors, using permeabilized T. gondii parasites. We have successfully used these methods to investigate the function of T. gondii proteins, including the apicomplexan parasite-specific protein subunit TgQCR11 of the coenzyme Q:cytochrome c oxidoreductase complex (ETC Complex III). We note that these methods are also amenable to screening compound libraries to identify candidate ETC inhibitors.
Background
Parasites in the phylum Apicomplexa cause a number of serious diseases in humans and other animals, including toxoplasmosis (Toxoplasma gondii) and malaria (Plasmodium species). Up to a third of the global human population is infected with T. gondii, making it one of the most successful parasites (Pappas et al., 2009). Several anti-parasitic compounds act by targeting the parasite mitochondrion (Alday and Doggett, 2017; Smith et al., 2021); for instance, the clinically used drug atovaquone inhibits the mitochondrial electron transport chain (ETC) (Fry and Pudney, 1992; Pfefferkorn et al., 1993; McFadden et al., 2000).
The ETC is composed of a series of multi-protein complexes (I-V) embedded in the inner mitochondrial membrane. The ETC functions to transport electrons derived from cellular metabolism through ETC Complexes I-IV to the final electron acceptor, oxygen. In mammalian cells, numerous dehydrogenases, including a large multimeric NADH dehydrogenase complex (Complex I) and a succinate dehydrogenase complex (Complex II), oxidize their substrates and reduce coenzyme Q (CoQ), a molecule that functions as an electron shuttle in the inner mitochondrial membrane (Hayward and van Dooren, 2019). CoQ binds to the so called Qo site of Complex III of the ETC, donating two electrons to iron-based co-factors in this complex. In a process known as the Q-cycle, one electron is passed via the iron-sulfur cluster of the Rieske protein and the heme group of cytochrome c1 to the soluble intermembrane space protein cytochrome c, while the other is passed via heme groups of cytochrome b to the Qi site, where CoQ docks and accepts the electron (Mitchell, 1975). Reduced cytochrome c transports electrons to Complex IV, which ultimately consumes oxygen as the final electron acceptor. In many organisms, electron transport through Complexes I, III, and IV is coupled to translocation of protons from the mitochondrial matrix into the intermembrane space, generating a proton motive force across the inner mitochondrial membrane. This proton gradient is harvested by ATP synthase (Complex V) to generate ATP, as well as being important for mitochondrial processes such as protein import (Schmidt et al., 2010).
The ETC of apicomplexan parasites differs in numerous ways from that of mammalian hosts such as humans (Figure 1) (Hayward and van Dooren, 2019). For instance, the large multimeric ETC Complex I found in mammalian cells is missing in T. gondii, and is replaced instead by single subunit NADH dehydrogenases (termed NDH2-I and NDH2-II), which are able to feed electrons from NADH to CoQ but do not contribute to the proton gradient (Saleh et al., 2007; Lin et al., 2008). The genome of T. gondii encodes additional dehydrogenases that oxidize substrates such as malate, glycerol 3-phosphate, dihydroorotate, and succinate, in each instance donating the electrons directly to CoQ (Figure 1). As in mammals, electron transport through Complexes III and IV in the parasite is coupled to proton translocation across the mitochondrial inner membrane, and oxygen is consumed as the terminal electron acceptor at Complex IV. However, the ETC complexes III, IV and V of T. gondii and related parasites contain many additional subunits that are not found in their mammalian counterparts (Huet et al., 2018; Seidi et al., 2018; Salunke et al., 2018; Evers et al., 2021; Hayward et al., 2021; Maclean et al., 2021; Mühleip et al., 2021). Furthermore, the CoQ binding sites of Complex III are sufficiently different from the equivalent sites in the mammalian complex to serve as the targets for anti-parasitic drugs, such as atovaquone and endochin-like quinolones (Fry and Pudney, 1992; Doggett et al., 2012; Fisher et al., 2020). There are currently few options available to assess ETC function in apicomplexan parasites. Those previously used include determination of O2 consumption using a Clark electrode (Vercesi et al., 1998), measurements of mitochondrial membrane potential using dyes such as rhodamine 123 (Divo et al., 1985), 3,3′dihexyloxacarbocyanine iodide (Srivastava et al., 1997), safranin O (Vercesi et al., 1998), and JC-1 (Brooks et al., 2010), and spectrophotometric assays to measure cytochrome c reduction (Fry and Beesley, 1991). Therefore, developing new methods to assess parasite ETC function are of interest both to study novel aspects of parasite biology/metabolism and to identify new anti-parasitic compounds.

Figure 1. The mitochondrial electron transport chain of T. gondii parasites. Dehydrogenases (pink) of the inner mitochondrial membrane (IMM) – namely, glycerol 3-phosphate dehydrogenase (G3PDH), dihydrooratate dehydrogenase (DHODH), succinate dehydrogenase (SDH; Complex II), malate:quinone oxidoreductase (MQO) and type 2 NADH dehydrogenases (NDH2) – donate electrons to coenzyme Q (CoQ; yellow) via oxidation of their substrates. CoQ interacts with ubiquinone:cytochrome c oxidoreductase (Complex III; orange) at the Qo and Qi sites in a process called the Q cycle. These interactions transfer electrons from CoQ to a soluble protein of the intermembrane space (IMS) called cytochrome c (CytC, dark red), as well as translocating protons (H+) from the mitochondrial matrix (MM) into the IMS. CytC donates electrons to cytochrome c oxidase (Complex IV; green), which consumes oxygen as the terminal electron acceptor and also contributes to the proton gradient across the IMM. The proton motive force generated by Complexes III and IV is harnessed by adenosine triphosphate (ATP) synthase (Complex V; purple), which couples the rotation of the F0 domain (caused by movement of protons from the IMS back into the MM) to ATP synthesis at the F1 catalytic domain. Modified from Hayward and van Dooren (2019).
Given that the primary cellular process that utilizes oxygen is the mitochondrial ETC, many methods used to assess ETC function measure changes in the concentration of dissolved oxygen in the assay medium, thereby calculating the oxygen consumption rate (OCR) of cells or isolated mitochondria. A traditional way to measure OCR is by polarography using a Clark electrode (Clark et al., 1953), which uses an electrochemical oxygen electrode to measure changes in the dissolved oxygen concentration in a suspension of cells/mitochondria, one sample at a time, in a closed chamber. A more recent technology, the Seahorse XFe96 analyzer, uses two solid state optical sensor probes to simultaneously measure the dissolved oxygen and proton concentration (i.e., the pH) in the assay medium surrounding cells in a high-throughput 96 well plate format, to derive the OCR and the extracellular acidification rate (ECAR), respectively. The sensor cartridge contains four ports above each well that enable the injection of compounds (e.g., substrates or inhibitors) at predetermined time points during the assay. ECAR is typically interpreted as a measure of glycolytic activity, since the extrusion of lactate (an end product of glycolysis) from cells is coupled with the extrusion of a proton, thus acidifying the culture medium (Zeng et al., 2021). However, as other metabolic pathways that produce and extrude H+ into the medium also contribute to ECAR (Moreno et al., 1998; Mookerjee et al., 2015), the interpretation that ECAR only assesses glycolytic activity is probably an oversimplification. The Seahorse XFe96 analyzer has been used successfully to assess mitochondrial function in several protozoan parasites, including Plasmodium falciparum (Sakata-Kato and Wirth, 2016) and Trypanosoma cruzi (Shah-Simpson et al., 2016).
Recent studies in our lab have used the Seahorse XFe96 system to measure the OCR and ECAR of intact extracellular T. gondii parasites, and thereby characterized the function of novel parasite-specific subunits of ETC Complexes III (Hayward et al., 2021), IV (Seidi et al., 2018), and V (Huet et al., 2018) (Figure 2). We have also utilized these assays to measure the effects of the loss of biosynthetic pathways for co-factors and prosthetic groups that play key roles in the ETC, such as heme (Tjhin et al., 2020) and iron-sulfur clusters (Aw et al., 2021). Additionally, we have developed methods to pinpoint the site of ETC dysfunction by assessing the OCR of permeabilized parasites supplied with different ETC substrates (Hayward et al., 2021). We note that these methods are also amenable to screening compound libraries for identifying inhibitors of the ETC, and for determining the targets of hit compounds. Here, we describe these experimental methods in detail as useful tools to investigate and dissect mitochondrial ETC function in T. gondii parasites.
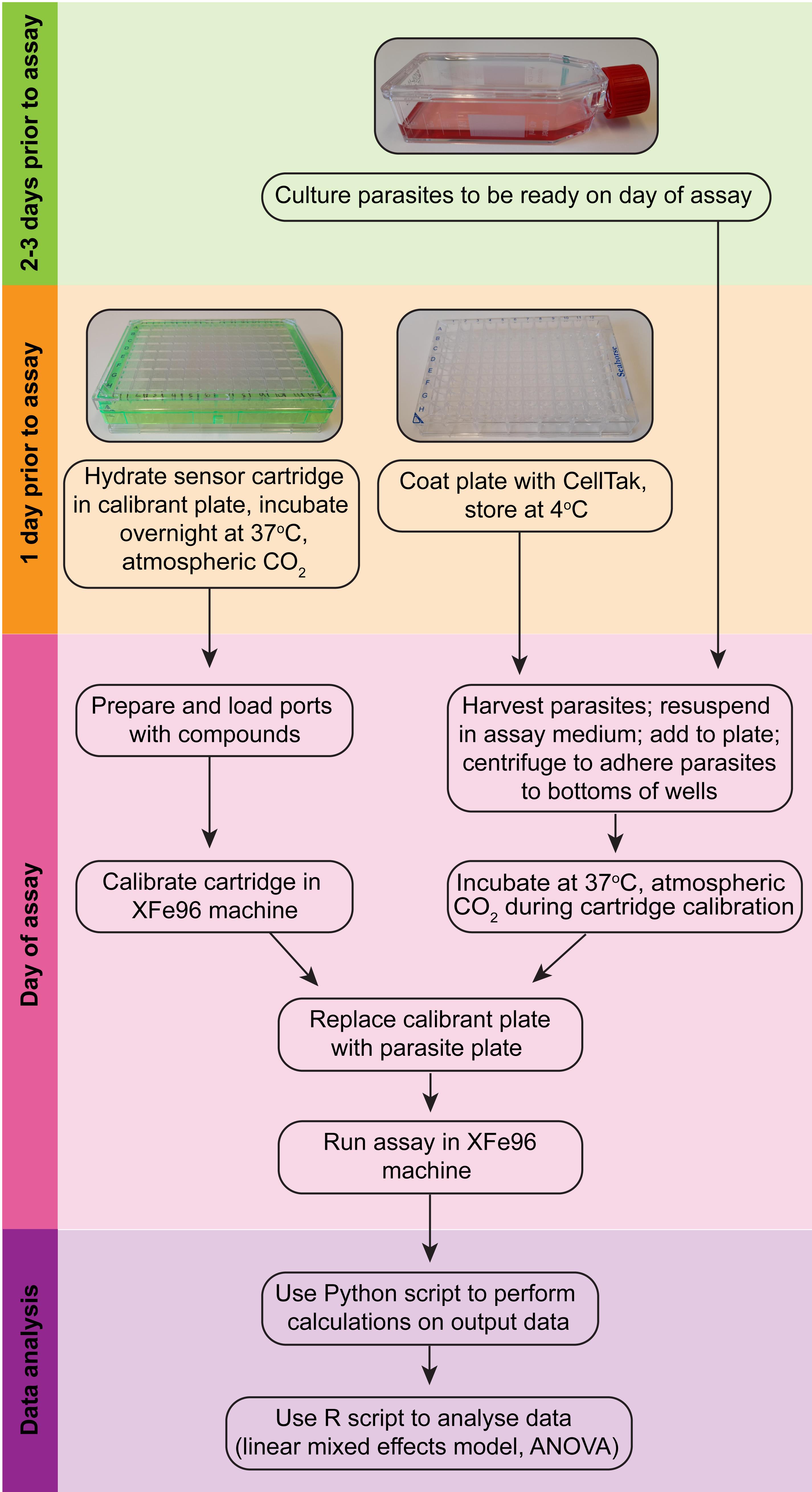
Figure 2. Overview of the Seahorse XFe96 assay procedure (modelled after Plitzko and Loesgen, 2018).
Materials and Reagents
25 cm2 tissue culture flasks (Greiner Bio-One, catalog number: 690175)
Sterile filter tips (20, 200 and 1,250 μL) (Interpath, catalog numbers: 24500, 24700, 24900)
Sterile serological pipettes (10, 25 mL) (Sigma, catalog numbers: CLS4489-200EA, CLS4488-200EA)
Sterile 2 mL aspiration pipettes (Greiner Bio-One, catalog number: 710183)
Cell scrapers (Sarstedt, catalog number: 83.3951)
Needle hypodermic 26 G × 13 mm (Terumo, catalog number: NN.2613R)
10 mL Luer Lock syringes (Terumo, catalog number: SS10L)
Millex-SV syringe filter unit 5 μM PVDF (Millipore, catalog number: SLSV025LS) or 25 mm Whatman Nuclepore 3 µm polycarbonate membrane filters (Cytiva, catalog number: 10418306). The 3 µm polycarbonate filters must be assembled into reusable 25 mm Whatman polypropylene Swin-Lok filters (Cytiva, catalog number: 420200).
Polypropylene centrifuge tubes (15, 50 mL) (Greiner Bio-One, catalog numbers: 188271, 227261)
Sterile Eppendorf tubes (1.5 mL) (Axygen, catalog number: MCT-150-C)
Reagent reservoirs (Sigma, catalog number: R1400)
Seahorse XF96 FluxPak (culture plates, sensor cartridge, calibrant; Agilent, catalog number: 102416-100)
Seahorse XF base medium (Agilent, catalog number: 102353-100)
Sodium bicarbonate (Sigma, catalog number: S6297-1KG)
Cell-Tak adhesive (Corning, catalog number: 354241)
L-glutamine (Sigma, catalog number: G8540-100G)
D-glucose (BDH AnalaR, catalog number: 101174Y)
Potassium hydroxide (AJAX-Finechem Univar, catalog number: AJA405-500G)
Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP) (Sigma, catalog number: C2920-10MG)
Dimethyl sulfoxide (DMSO) (Sigma, catalog number: D8418-250G)
Digitonin (Sigma, catalog number: D141-500MG)
L-malic acid (Sigma, catalog number: M1000-100G)
sn-glycerol 3-phosphate bis(cyclohexylammonium) salt (Sigma, catalog number: G7886-1G)
Atovaquone (Sigma, catalog number: A7986-10MG)
N,N,N,N’-tetramethyl-p-phenylenediamine (TMPD) (Sigma, catalog number: T7394-5G)
L-ascorbic acid (Sigma, catalog number: A5960-25G)
Sodium azide (AJAX-Finechem Univar, catalog number: AJA1222-100G)
Ethylene glycol-bis(2-amino-ethylether)-N,N,N,N’-tetraacetic acid (EGTA) (Sigma, catalog number: E3889-25G)
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (Sigma, catalog number: H3375-250G)
Potassium phosphate monobasic (KH2PO4) (ChemSupply, catalog number: PA009-500G)
Magnesium chloride hexahydrate (MgCl2·6H2O) (AJAX-Finechem Univar, catalog number: AJA296-500G)
D-mannitol (Sigma, catalog number: M9546-250G)
Sucrose (Sigma, catalog number: S9378-5KG)
Essentially fatty acid free bovine serum albumin (FAF-BSA) (Sigma, catalog number: A3803-10G)
MilliQ H2O
Sodium bicarbonate (0.1 M) (see Recipes)
Glutamine stock (200 mM) (see Recipes)
Glucose stock (1 M) (see Recipes)
Base medium with glucose (5 mM) and glutamine (1 mM) (BM+Glc+Gln) (see Recipes)
FCCP stock (50 mM) (see Recipes)
Atovaquone stock (10 mM) (see Recipes)
Digitonin stock (5% w/v) (see Recipes)
Mitochondrial Assay Solution (MAS) buffer (1×, 500 mL) (see Recipes)
Malate stock (320 mM) (see Recipes)
Glycerol 3-phosphate stock (200 mM) (see Recipes)
TMPD stock (500 mM) (see Recipes)
Ascorbate stock (200 mM) (see Recipes)
Sodium azide stock (110 mM) (see Recipes)
Equipment
Pipettes 0.1-2.5 μL, 2-20 μL, 20-200 μL and 100-1,000 μL (Eppendorf, catalog numbers: 3120000011, 3120000038, 3120000054, 3120000062)
Multichannel pipettes 10-100 μL and 30-300 μL (Eppendorf, catalog numbers: 3122000035, 3122000051)
Seahorse XFe96 Analyzer (Agilent)
Humidified, 37°C, non-CO2 incubator
Humidified, 37°C, 5% CO2 incubator
Hemocytometer, Improved Neubauer bright line cell counting chamber (Thermo Fisher Scientific, 81002.04)
Standard inverted light microscope (Olympus CKX41), fitted with 4×, 10×, 20× and 40× objective lenses
Biological Safety Cabinet Class II
Aspirator vacuum pump
Water bath, 37°C
4°C fridge and -20°C freezer
pH meter
Software
Wave Software (Agilent)
Excel (Microsoft)
Python (version 3.7 or higher)
R (version 4.0.5 or higher)
GraphPad Prism (GraphPad)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Hayward, J. A., Rajendran, E., Makota, F. V., Bassett, B. J., Devoy, M., Neeman, T. and van Dooren, G. G. (2022). Real-Time Analysis of Mitochondrial Electron Transport Chain Function in Toxoplasma gondii Parasites Using a Seahorse XFe96 Extracellular Flux Analyzer. Bio-protocol 12(1): e4288. DOI: 10.21769/BioProtoc.4288.
分类
微生物学 > 微生物细胞生物学 > 基于细胞的分析方法 > 报告基因实验
细胞生物学 > 基于细胞的分析方法 > 线粒体呼吸
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










