- EN - English
- CN - 中文
Measurement of Reactive Oxygen and Nitrogen Species in Living Cells Using the Probe 2',7'-Dichlorodihydrofluorescein
使用探针 2',7'-二氯二氢荧光素测量活细胞中的活性氧和氮种类
发布: 2021年12月20日第11卷第24期 DOI: 10.21769/BioProtoc.4279 浏览次数: 3870
评审: ASWAD KHADILKARManasi K. MayekarArvind Panday

相关实验方案

在平板检测仪中使用高特异性荧光探针定量巨噬细胞细胞二价铁 (Fe2+) 含量
Philipp Grubwieser [...] Christa Pfeifhofer-Obermair
2024年02月05日 2226 阅读

哺乳动物线粒体和胞质顺乌头酸酶的凝胶内活性测定——分区特异性氧化应激与铁状态的替代标志物
Wing-Hang Tong and Tracey A. Rouault
2024年12月05日 2208 阅读
Abstract
Reactive oxygen species and reactive nitrogen species (RONS) are involved in programmed cell death in the context of numerous degenerative and chronic diseases. In particular, the ability of cells to maintain redox homeostasis is necessary for an adaptive cellular response to adverse conditions that can cause damage to proteins and DNA, resulting in apoptosis and genetic mutations. Here, we focus on the 2',7'-dichlorodihydrofluorescein diacetate (DCFH2-DA) assay to detect RONS. Although this fluorescence-based assay is widely utilized due to its high sensitivity to detect changes in cellular redox status that allow measuring alterations in RONS over time, its validity has been a matter of controversy. If correctly carried out, its limitations are understood and results are correctly interpreted, the DCFH2-DA assay is a valuable tool for cell-based studies.
Background
Reactive oxygen and nitrogen species (RONS) is a shared term for oxygen-based radicals and/or chemically reactive oxidants including nitric oxide (•NO), superoxide (O2•−), hydrogen peroxide (H2O2), peroxynitrite (ONOO−), hypochlorous acid (HOCl), and hydroxyl radical (•OH), among others (Halliwell, 2012). RONS, produced mainly from nicotinamide adenine dinucleotide phosphate oxidase and nitric oxide synthase, play an essential role in signaling cascades regulating cellular processes such as proliferation, differentiation, motility, and programmed cell death (Dröge, 2002). Of note, in view of a cellular vulnerability to oxidants or activators of oxidative metabolism that can induce cell death, an usually tightly regulated steady-state control over the production-detoxification of RONS protects cells from their harmful effects (Harris and Brugge, 2015). This has stimulated increased investigations and lead to tools that are aimed at a better understanding of the biochemistry and physiology of redox reactions of the RONS. However, these tools are not able to assess all facets of redox mechanisms, specifically in living cells and organisms, since detection and quantification of short-lived RONS requires practices that respond to them very rapidly, as opposed to classical antioxidants that finally generate stable products (Dikalov and Harrison, 2014; Brandes et al., 2018). While RONS and redox biology are associated with complex systems, assays that are used for the measurement of RONS involved in biological systems should be approached with caution.
Fluorescent probes produce stable products upon reactions with RONS and can offer high sensitivity detection (Hempel et al., 1999). Redox signaling mechanisms can be explored using 2',7'- dichlorodihydrofluorescein diacetate (DCFH2-DA); this cell-permeable ester is hydrolyzed inside the cell to 2',7'-dichlorodihydrofluorescein (DCFH2) by cytosolic esterases, which causes the probe’s retention in the cell to allow detection of intracellular oxidizing reactions (Figure 1). In the presence of RONS, DCFH2 is oxidized to 2',7'-dichlorofluorescein (DCF) that serves as the fluorescent indicator and can be easily determined, providing a simple and sensitive analytical method for detecting intracellular oxidants (Glebska and Koppenol, 2003). However, oxidation of DCFH2 in cellular systems reflects not only the rate of production of oxidants and the rate constants for the critical one-electron oxidation step but also other cell-specific and context-dependent aspects. For example, ubiquitous cellular antioxidants such as glutathione (Figure 2) and other protein thiols or antioxidant moieties will attenuate the generation of fluorescence, dependent on the overall redox status (Albrecht et al., 2011). Moreover, despite the popularity of DCFH2 to assess oxidative stress, the identity of oxidant species responsible remains largely unclear. The correct use and interpretation of the DCFH2 assay require knowledge about contraindications or pitfalls. Notably, DCFH2 may be a valuable and sensitive indicator of cellular toxicity, but novel generations of probes must provide information about the exact cellular localization of the oxidizing RONS, reaction rates with different types of RONS, kinetics of the metabolism or decomposition of the oxidized probe, and correlation with downstream signaling (Halliwell and Whiteman, 2004; Winterbourn, 2008; Murphy et al., 2011). Carefully attention to these facts will eliminate erroneous interpretations and help to move exciting approaches of free radical research forward.
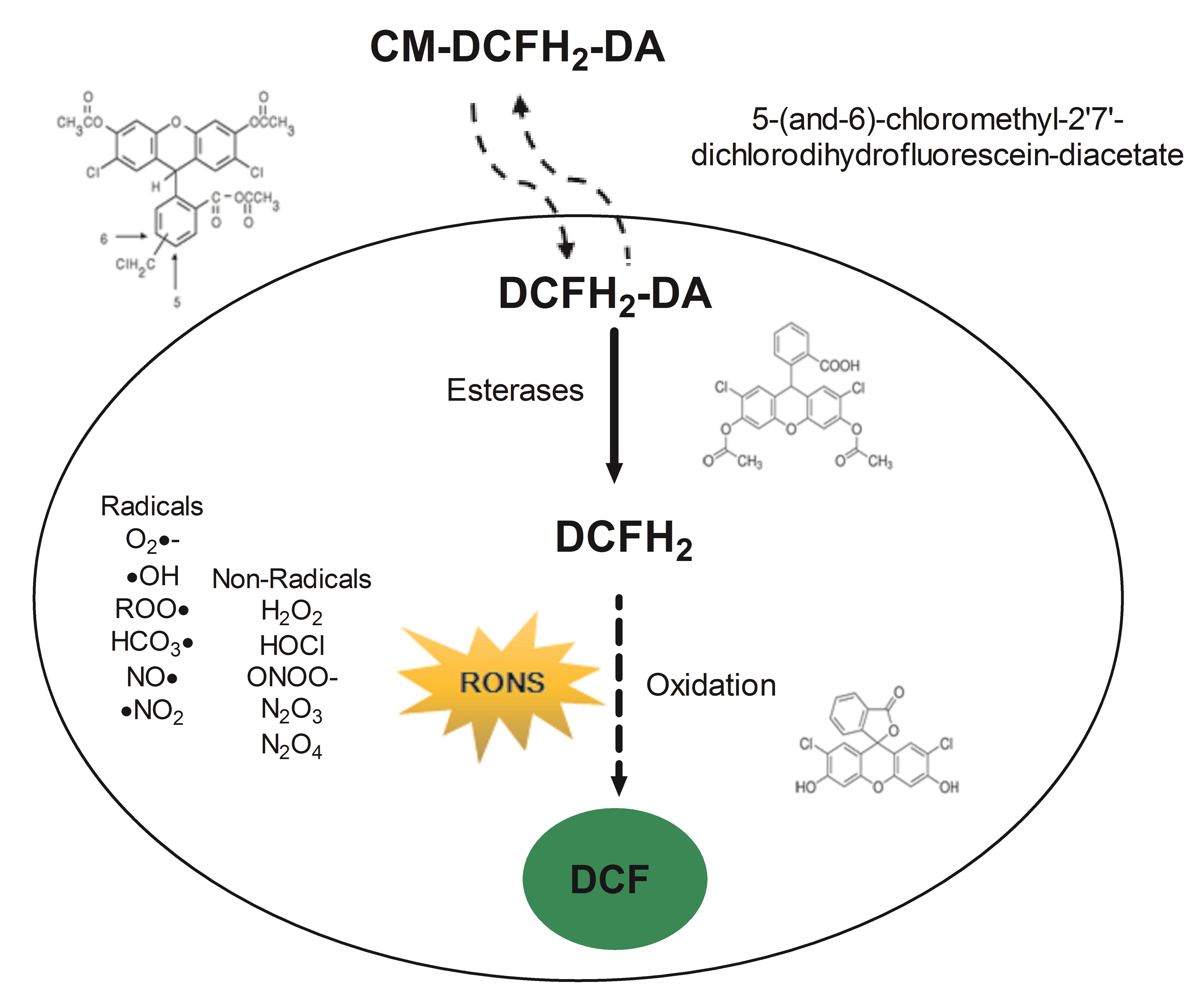
Figure 1. Schematic overview of the workflow described in this protocol. CM-DCFH2-DA diffuses into the cell, intracellular esterases hydrolyze the acetate groups, generating 2',7'- dichlorodihydrofluorescein (DCFH2) that reacts with intracellular oxidants resulting in the observable highly fluorescent compound 2',7'- dichlorofluorescein (DCF).
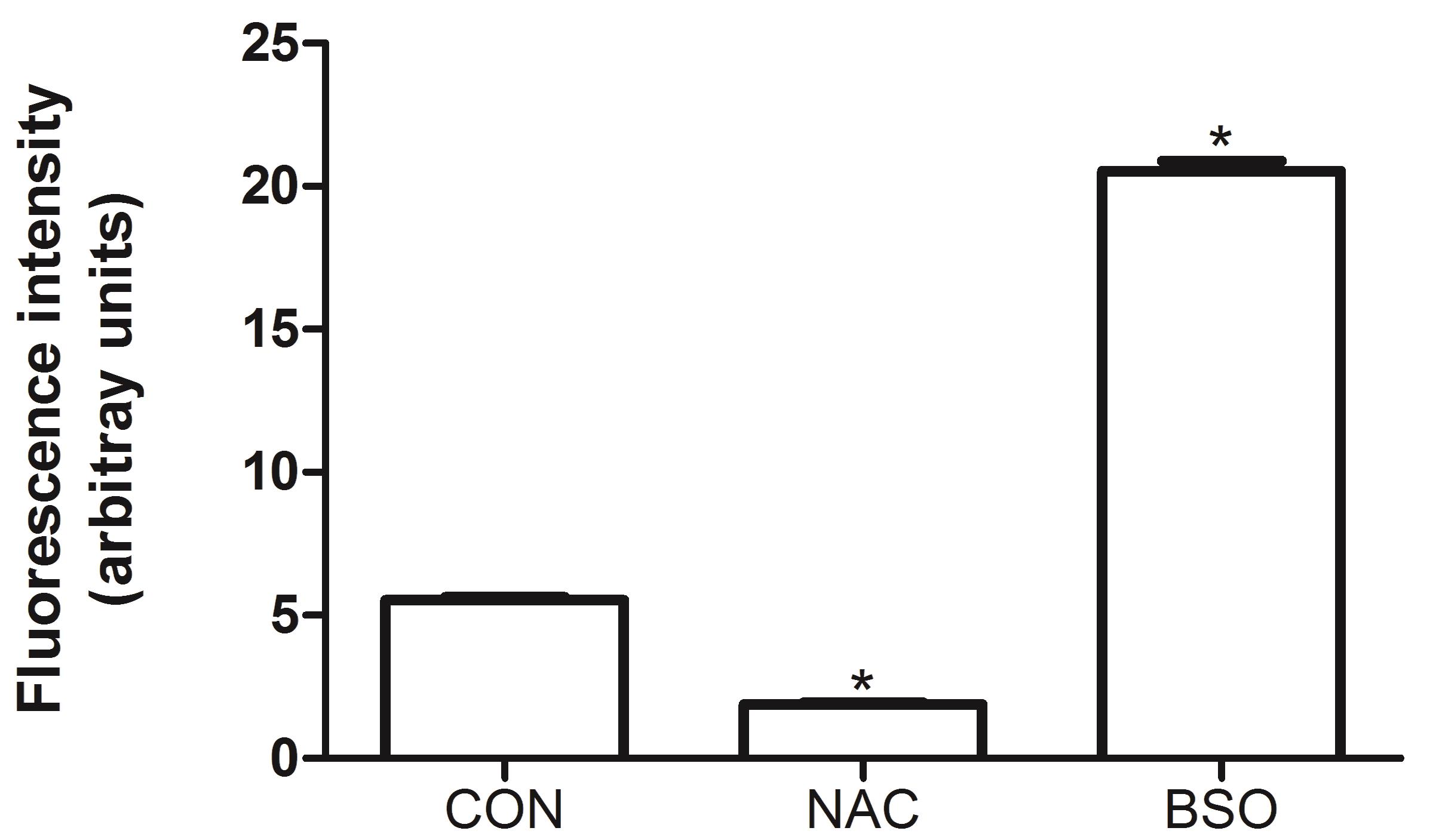
Figure 2. Effect of N-acetylcysteine (NAC) and buthionine sulfoximine (BSO) on the formation of DCF. The antioxidant NAC (5 mM) and the pro-oxidant BSO (10 µM), which significantly deplete total glutathione levels by inhibiting γ-glutamyl-cysteine synthetase (a key enzyme in glutathione biosynthesis), were evaluated on human umbilical vein endothelial cells (HUVEC) showing extremes of low and high oxidation of DCFH2, respectively. HUVEC cells were seeded at the density of 2.0 × 106 cells/cm2 for 24 h. BSO and NAC were preincubated for 1 h. The oxidation of DCFH2 in NAC or BSO treated HUVEC was compared with that of untreated control (CON) cells. The measurements of fluorescence were executed at room temperature. Data were analyzed by one-way analysis of variance with Dunnett’s Multiple Comparison Test. The data represent the means ± SEM. *P < 0.05 versus CON.
Materials and Reagents
CM-DCFH2-DA (Invitrogen Life Technologies/Molecular Probes, EUA), storage temperature at -20°C
High quality anhydrous dimethylsulfoxide (DMSO), dimethyl-formamide (DMF) or 100% ethanol, H2O2, and organic salts (Merck, Brazil), storage in 15/30°C
Loading buffer, such as a simple physiological buffer (PBS), storage in 4°C fridge
N-acetyl-L-cysteine (NAC) (Sigma, catalog number: A7250), storage in 4°C fridge
Buthionine Sulfoximine (BSO) (Sigma, catalog number: B2515), storage in 4°C fridge
HUVEC were obtained from the American Type Culture Collection, cultivated in RPMI 1640 medium supplemented with 10% fetal bovine serum, penicillin, and streptomycin antibiotics. Experiments were performed at 37°C and in a humidified atmosphere of 95% air and 5% CO2.
PBS (see Recipes)
Phosphate buffer (see Recipes), storage in 4°C fridge
Equipment
Spectrophotometer model U-2010 (Software UV Solutions, version 1.1, Hitachi, Japan)
96-well microplates (black-walled, clear bottom; Corning, catalog number: CLS3829)
Software
GraphPad Prism Version 5.01 (or higher) (GraphPad Software Inc.)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Dornas, W. and Schuppan, D. (2021). Measurement of Reactive Oxygen and Nitrogen Species in Living Cells Using the Probe 2',7'-Dichlorodihydrofluorescein. Bio-protocol 11(24): e4279. DOI: 10.21769/BioProtoc.4279.
分类
发育生物学 > 细胞信号传导 > 胁迫反应
医学
细胞生物学 > 细胞新陈代谢 > 其它化合物
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link