- EN - English
- CN - 中文
Chronic Daily House Dust Mite Exposure in Mice is an Effective Model to Quantify the Effect of Pharmacologic Agents on Discrete Stages of Artery Remodeling in Pulmonary Hypertension
小鼠每日慢性房尘螨暴露是一种量化药物对肺动脉高压动脉重塑离散阶段影响的有效模型
发布: 2022年01月05日第12卷第1期 DOI: 10.21769/BioProtoc.4273 浏览次数: 4907
评审: Manjula MummadisettiDavide BottaAnonymous reviewer(s)
Abstract
Pulmonary hypertension (PH) is a heterogenous and incurable disease marked by varying degrees of pulmonary vascular remodeling. This vascular remodeling, which includes thickening of the smooth muscle layer (an early finding) and formation of occlusive neointimal lesions (a late finding) in the pulmonary arteries, is a major driver of morbidity and mortality in PH. Available PH therapies consist of vasodilators that do not specifically target lesion formation or expansion and neither prevent progression nor reverse disease. This paucity of curative treatments highlights the need for new drug discovery targeting crucial steps of artery remodeling in PH. The cell dynamics and molecular signals driving neointimal lesion formation have been difficult to elucidate as classic mouse models of PH do not develop neointima. Here, we detail the methods to generate a robust and non-genetic mouse model of PH with medial thickening and neointimal lesion formation in the pulmonary arteries, through chronic exposure to an inflammatory stimulus—house dust mite (HDM). This model rapidly generates human-like pulmonary arterial lesions following a reproducible time course, allowing scrutiny of the cellular and molecular mechanisms controlling each stage of artery remodeling. Further, we outline optimal tissue handling, sectioning, and staining methodologies for detailed quantitative analysis of artery medial thickening and neointimal lesion formation and expansion. Finally, we present a method for staged pharmacologic intervention to identify molecules and pathways required at each step of the pulmonary arterial remodeling process. The advantages of this mouse model of PH over currently available animal models are five-fold. (i) It allows the use of the full range of genetic and single cell tools available in mice to manipulate and study the process of vascular remodeling seen in human disease, including the formation of neointimal lesions in a controlled and cell specific manner. (ii) The vascular lesions develop in a stereotyped manner with predictable timing, allowing for pharmacologic manipulation at discrete stages of vessel remodeling. (iii) It is rapid, with development of PH and vascular remodeling in a timeframe of two to eight weeks. (iv) It uses simple techniques and requires neither surgery, unusual equipment, or extensive personnel training. (v) The staining and quantitation methodologies we present are a significant improvement over those currently in use in the field. We hope that dissemination of this model and the associated detailed methods will speed up the development of novel and more effective PH therapeutics.
Graphic abstract:
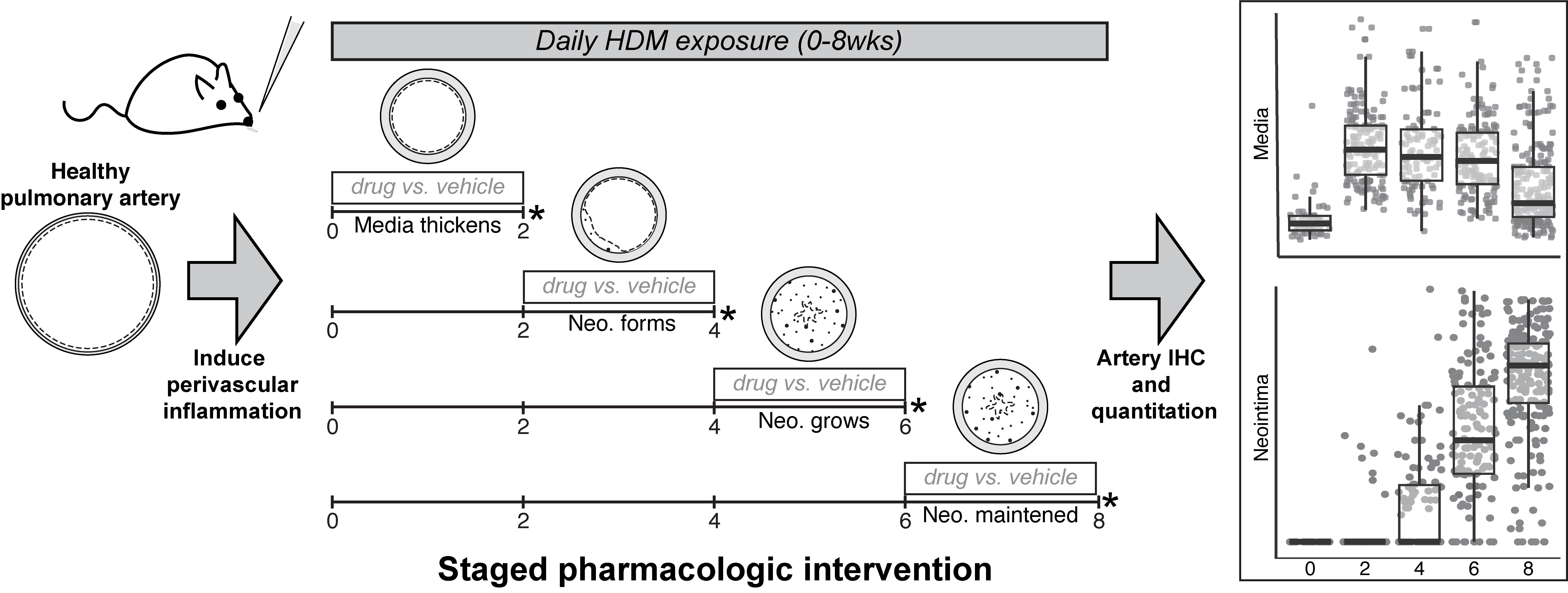
Chronic perivascular inflammation induces medial thickening and neointima formation in pulmonary arteries, following a stereotyped time course, and allowing staged pharmacologic intervention during specific remodeling events, as well as quantitative assessment of vascular changes.
Background
Pulmonary hypertension (PH) is a fatal disease affecting children and adults with no available cure. It affects patients of all ages and is estimated to result in 200,000 hospitalizations and 15,000 deaths each year (Lau et al., 2017). PH encompasses a heterogenous group of pathologic processes that result in elevation in pulmonary arterial pressures (Simonneau et al., 2019). In all forms of PH, there is prominent vascular remodeling of the arteries of the pulmonary circulation, with thickening and contraction of the medial smooth muscle layer followed by the formation of occlusive ‘neointimal lesions’ in medium and small pulmonary arteries. These pathological changes narrow vessel lumens (Figure 1), increase pulmonary vascular resistance, and ultimately result in right heart failure and death (Humbert et al., 2018).
Available PH therapies consist of pulmonary vasodilators which do not specifically target artery remodeling and do not prevent progression or reverse disease (Spiekerkoetter et al., 2019). Current mouse models either reproduce only limited features of the disease (Stenmark et al., 2006), or are driven by multi-allelic genetic approaches (Dai et al., 2016) that limit the use of additional genetic tools to study disease processes (Gomez-Arroyo et al., 2012). A non-genetically driven murine model of pulmonary hypertension, with robust pulmonary vascular changes, should allow a granular analysis of the cellular and molecular basis of artery remodeling in PH, and could facilitate the development of new more effective therapies.
Here, we present a detailed protocol for inducing PH in mice that reproduces human vasculopathy in a tractable and reproducible time frame, with simple non-genetic methods. The vascular changes observed in this model closely mimic those observed in PH patients, and include medial thickening, formation and expansion of occlusive neointimal lesions, elaboration of the bronchial circulation, and muscularization of the veins (Steffes et al., 2020). Most notably, pulmonary artery remodeling in this model occurs in three temporally distinct steps, beginning with medial thickening, followed by establishment of small neointimal lesions, and finally rapid proliferation of neointimal cells to occlude the lumen, shown in Figure 2A-2B. These stages occur during well-defined temporal windows, providing a tractable framework through which to quantify the effect of pharmacologic or genetic manipulation during discrete events in artery remodeling. Additionally, we provide tissue preparation, staining, and quantification protocols to visualize key elements of arterial lesions with multicolor fluorescence microscopy highlighting elastin, smooth muscle, neointima, and endothelium.
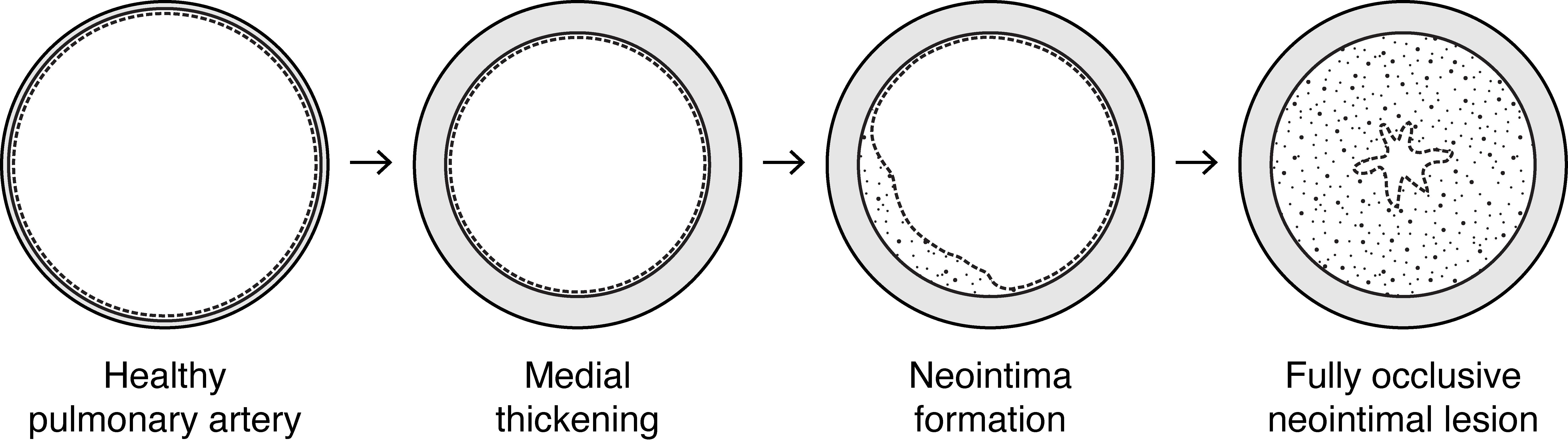
Figure 1. Progressive artery remodeling in human pulmonary arterial hypertension (PAH). Pulmonary arterial hypertension is marked by progressive remodeling of small to medium sized pulmonary arteries and arterioles. Healthy arteries have a thin medial smooth muscle cell layer (grey) located between the internal and external elastic laminae (black solid lines) and a lumen lined with endothelium (dashed line). In early PAH and other forms of PH there is thickening of the medial smooth muscle cell layer. Later in disease, neointimal lesions (white speckled area) form between the internal elastic lamina and the endothelium and grow to occlude the vessel lumen in end stage disease.
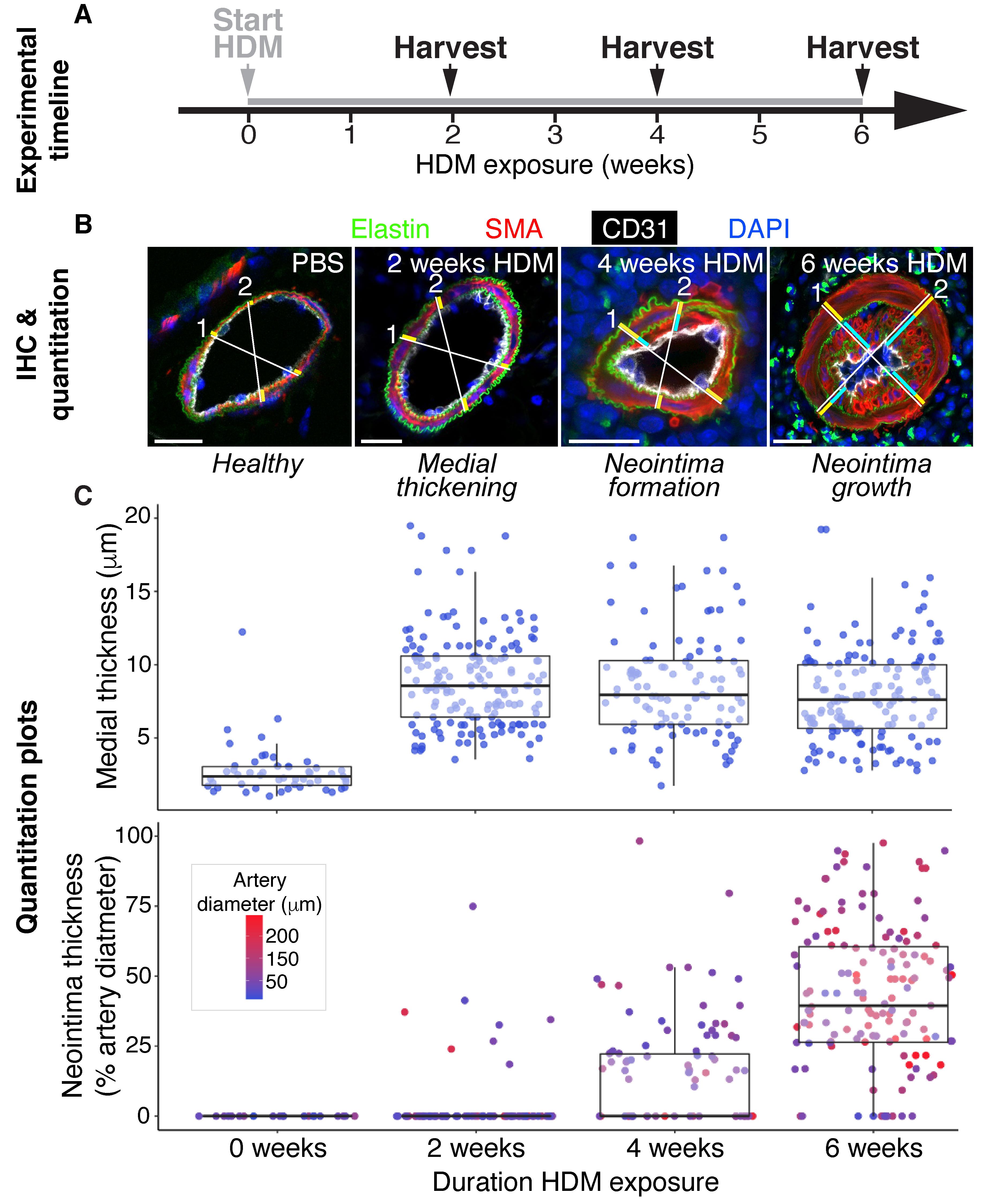
Figure 2. Protocol overview summarizing experimental timeline, immunohistochemistry (IHC), and vessel remodeling quantification. A-B. Daily intranasal HDM exposure in mice induces discrete stages of pulmonary arterial remodeling over 6 weeks, with progression from healthy arteries prior to HDM administration, to medial thickening seen in animals harvested at 2 weeks, neointimal formation after 4 weeks, and diffuse neointimal lesion growth and luminal occlusion by 6 weeks. B. Confocal micrographs of IHC performed on 20 μm cryosections with the optimized staining protocol outlined in Table 1 allow detailed quantification of artery remodeling, by clearly visualizing the internal and external elastic laminae (elastin/hydrazide, green), the medial smooth muscle cell layer [smooth muscle actin (SMA)+ cells, red, between the internal and external elastic laminae], the endothelium (CD31, white), neointimal lesions (SMA+ cells between the internal elastic lamina and the endothelium), and nuclei (DAPI, blue). To quantitate degree of vessel remodeling, two orthogonal measurement axes are drawn per vessel, and their length in micrometer are recorded and represent external vessel diameter (white lines), medial thickness (yellow bars), and neointimal thickness, when present (light blue bars). C. Vessel quantitation measurements for ~50 vessels per timepoint are displayed in scatterplots, with values grouped by duration of HDM exposure. Absolute medial thickness (top plot) and neointimal thickness expressed as percentage of vessel diameter (bottom plot) are useful ways to visualize the data. The media thickens during the first two weeks of HDM exposure, and remains thickened through the subsequent 4 weeks of HDM exposure. Neointima is established after 4 weeks HDM exposure, and undergoes rapid expansion during weeks 4-6. Each dot represents a discrete vessel measurement. Dot color in the bottom plot reports vessel diameter. Scale bars in B are 20 μm.
Materials and Reagents
Animals
Eight to ten-week-old BALB/c female mice (Charles River, strain 028)
House dust mite preparation and administration
1.5 mL Eppendorf polypropylene tube with locking cap (Fisher Scientific, catalog number: 05-402-25)
200 μL pipette (Millipore Sigma, Eppendorf, catalog number: Z740441)
200 μL pipette filter tips (Fisher Scientific, catalog number: 05-413-952)
Isoflurane (VetOne, catalog number: 502017)
Lung dissection, perfusion, and inflation
70% ethanol (Fisher Scientific, catalog number: NC9663244)
Spray bottle (Fisher Scientific, catalog number: 02-991-721)
50 mL syringe (Fisher Scientific, catalog number: 14-817-57)
Butterfly needle, 27 G (Fisher Scientific, catalog number: 02-664)
Aluminum foil sheets (Fisher Scientific, catalog number: 01-213-106)
Styrofoam pad (e.g., lid of Styrofoam box, to use as dissection pad)
21 G 1” needles (to use as pins) (Fisher Scientific, catalog number: 14-840-92)
C-fold paper towels
Silk surgical suture thread (Fine Science Tools, catalog number: 18020-01)
5 mL syringe (Fisher Scientific, catalog number: 14-829-45)
20 G 0.5” blunt needle/cannula (Sai Infusion, catalog number: B20-50)
Falcon 50 mL conical tubes (Fisher Scientific, catalog number: 14-432-22)
Falcon 15 mL conical tubes (Fisher Scientific, catalog number: 14-959-53A)
Ice
90 mm Petri dishes (Fisher Scientific, catalog number: 07-202-028)
Water bath (Fisher Scientific, catalog number: 07-202-155)
10% neutral buffered formalin (Fisher Scientific, catalog number: 22-026-213)
Lab marker (Fisher Scientific, catalog number: 13-379-4)
Tissue Handling and Staining
90 mm Petri dishes (Fisher Scientific, catalog number: 07-202-028)
7.5 mL transfer pipettes (Fisher, catalog number: 13-711-47)
Falcon 15 mL conical tubes (Fisher Scientific, catalog number: 14-959-53A)
Optimal cutting temperature (OCT) compound (Sakura, catalog number: 4583)
25 mm × 20 mm × 5 mm specimen cryomolds (Fisher Scientfic, catalog number: NC9643511)
Biopsy tissue cassette (Sakura, catalog number: 4154-01)
Methanol wash bottle (×4) (Fisher Scientific, catalog number: 03-409-11H)
Superfrost+ microscope slides (25/75/1 mm) (Fisher Scientific, catalog number: 12-550-15)
Glass cover slips (24×60-1) (Fisher Scientific, catalog number: 12-545-MP)
Slide storage boxes (Fisher Scientific, catalog number: 14-372-86)
ImmEdge hydrophobic pen (Vector Labs, catalog number: H-4000)
1.5 mL Eppendorf polypropylene tube with locking cap (Fisher Scientific, catalog number: 05-402-25)
241 × 241 × 20 mm bioassay dish (Thermo Scientific, catalog number: 240845) with four 5 mL pipettes (Thermo Scientific, catalog number: 170373N) trimmed to size to fit horizontally across the interior of the dish, with two sets of two pipettes placed 50 mm apart and scotch taped in place as slide supports, and lined with moistened paper towels (shown in Figure 7C). Cover with aluminum foil for dark incubations.
Aluminum foil sheets (Fisher Scientific, catalog number: 01-213-106)
Antibodies and dyes (detailed in Table 1)
DAPI (cell nuclei) (Invitrogen, catalog number: D1306)
Hydrazide A488 (elastin) (Invitrogen, catalog number: A10436)
Mouse α-SMA Cy3 (SMCs & neointima) (Sigma, catalog number: C6198)
Armenian Hamster α-CD31 (endothelium) (Bio-Rad, catalog number: MCA1370Z)
Goat α-Armenian Hamster A647 (Invitrogen, catalog number: A-21451)
Prolong Gold firm setting mounting medium (Thermo Fisher, catalog number: P36930)
10× Phosphate buffered saline (PBS), diluted to 1× PBS in distilled water (dH2O) (Quality Biological, catalog number: 119-069-101). Store at room temperature (RT), and use within 12 months (see Recipes)
House dust mite (HDM) (Stallergenes Greer, catalog number: XPB70D3A25). Store at -20°C in 120 µL aliquots in PBS, and use within 12 months (see Recipes)
Heparin/NaCl in PBS. Store at 4°C, and use within 3 months (see Recipes)
NaCl (Millipore Sigma catalog number: S9888-500G)
Heparin (Millipore Sigma, catalog number: H3393)
2% low melting point (LMP) agarose in PBS (Fisher Scientific, catalog number: 16-520-050). Store at 4°C, and use within 3 months (see Recipes)
16% paraformaldehyde (PFA), diluted to 4% PFA in PBS (Electron Microscopy Sciences, catalog number: 15170. Store 16% PFA at RT, and use within 12 months. Keep 4% PFA solution on ice, and use the same day (see Recipes)
30% sucrose in PBS (Milipore Sigma, catalog number: S0389). Store at 4°C, and use within 1 month (see Recipes)
Methanol series, diluted with dH2O to the desired concentrations (Honeywell, catalog number: BB233-4). Store dilutions in wash bottles at RT, and use within 1 month (see Recipes)
PBST (0.1% Tween 20 in PBS) (Millipore Sigma, catalog number: 9005-64-5). Store at RT, and use within 1 month (see Recipes)
Preblock. Store at 4°C, and use within 24 h (see Recipes)
Triton X-100 (Millipore Sigma, catalog number: T8787)
Goat serum (Bio-Rad, catalog number: C07SA)
BSA (Millipore Sigma, catalog number: A2153)
Equipment
House dust mite preparation and administration
Anesthesia induction chamber (Harvard Apparatus, catalog number: 75-2029)
Air filter cannister (Harvard Apparatus, catalog number: 60-0979)
Oxygen tank, E 25cf (Praxair, catalog number: OX 2.6MC-E)
Isoflurane anesthesia machine (Fisher Scientific, catalog number: 50-154-0189)
Lung dissection, perfusion, and inflation
Blunt-tip curved serrated iris forceps (Fine Science Tools, catalog number: 11370-31)
Pointed-tip curved serrated forceps (Fine Science Tools, catalog number: 11270-20)
Dissection scissors (Fine Science Tools, catalog number: 14558-11)
Tissue Handling and Staining
Blunt-tip curved serrated iris forceps (Fine Science Tools, catalog number: 11370-31)
Dissection scissors (Fine Science Tools, catalog number: 14558-11)
Cryostat (Leica, model: CM3050S)
Vibratome (Leica, model: VT1000 S)
Humid chamber (homemade: assembly instructions above in “Materials and Reagents”, part D “Tissue Handling and Staining”, point 13, and in Figure 7C)
Software
Zen Blue or Zen Black (Carl Zeiss Microscopy)
Fiji/ImageJ (Imagej.net)
Google Sheets (google.com)
Excel (Microsoft)
R with R Studio (R-project.org; rstudio.com)
Ggplot2 (ggplot2.tidyverse.org)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Steffes, L. C. and Kumar, M. E. (2022). Chronic Daily House Dust Mite Exposure in Mice is an Effective Model to Quantify the Effect of Pharmacologic Agents on Discrete Stages of Artery Remodeling in Pulmonary Hypertension. Bio-protocol 12(1): e4273. DOI: 10.21769/BioProtoc.4273.
分类
医学 > 心血管疾病
细胞生物学 > 细胞信号传导 > 胁迫反应
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link














