- EN - English
- CN - 中文
A Simple and Straightforward Approach for Generating Small, Stable, Homogeneous, Unilamellar 1-Palmitoyl 2-Oleoyl Phosphatidylcholine (POPC) Bilayer Vesicles
一种生成小型、稳定、均质、单层 1-棕榈酰 2-油酰磷脂酰胆碱 (POPC) 双层囊泡的简单方法
发布: 2021年12月20日第11卷第24期 DOI: 10.21769/BioProtoc.4271 浏览次数: 3248
评审: Philipp A.M. Schmidpetersujan kumar mondalAnonymous reviewer(s)
Abstract
Various methods have been developed to generate phosphoglyceride liposomes. Approaches resulting in homogeneous populations of unilamellar bilayer vesicles are generally preferred to mimic various cell membrane situations, as well as to optimize aqueous solute trapping efficiency using the least amount of lipid for biotechnological purposes. Most are time-consuming, often tedious, or require specialized equipment, and produce vesicles with limited shelf-life at room temperature or in cold storage. Herein, we describe a straightforward approach that avoids the preceding complications and streamlines the construction of unilamellar bilayer vesicles from 1-palmitoyl-2-oleoyl phosphatidylcholine (POPC)/dihexanoyl phosphatidylcholine (DHPC) bicelle mixtures at room temperature. The resulting vesicles are small (32-36 nm diameter), unilamellar, bilayer vesicles that are homogeneous, stable, and resistant to freeze-thaw alterations.
Graphic abstract:
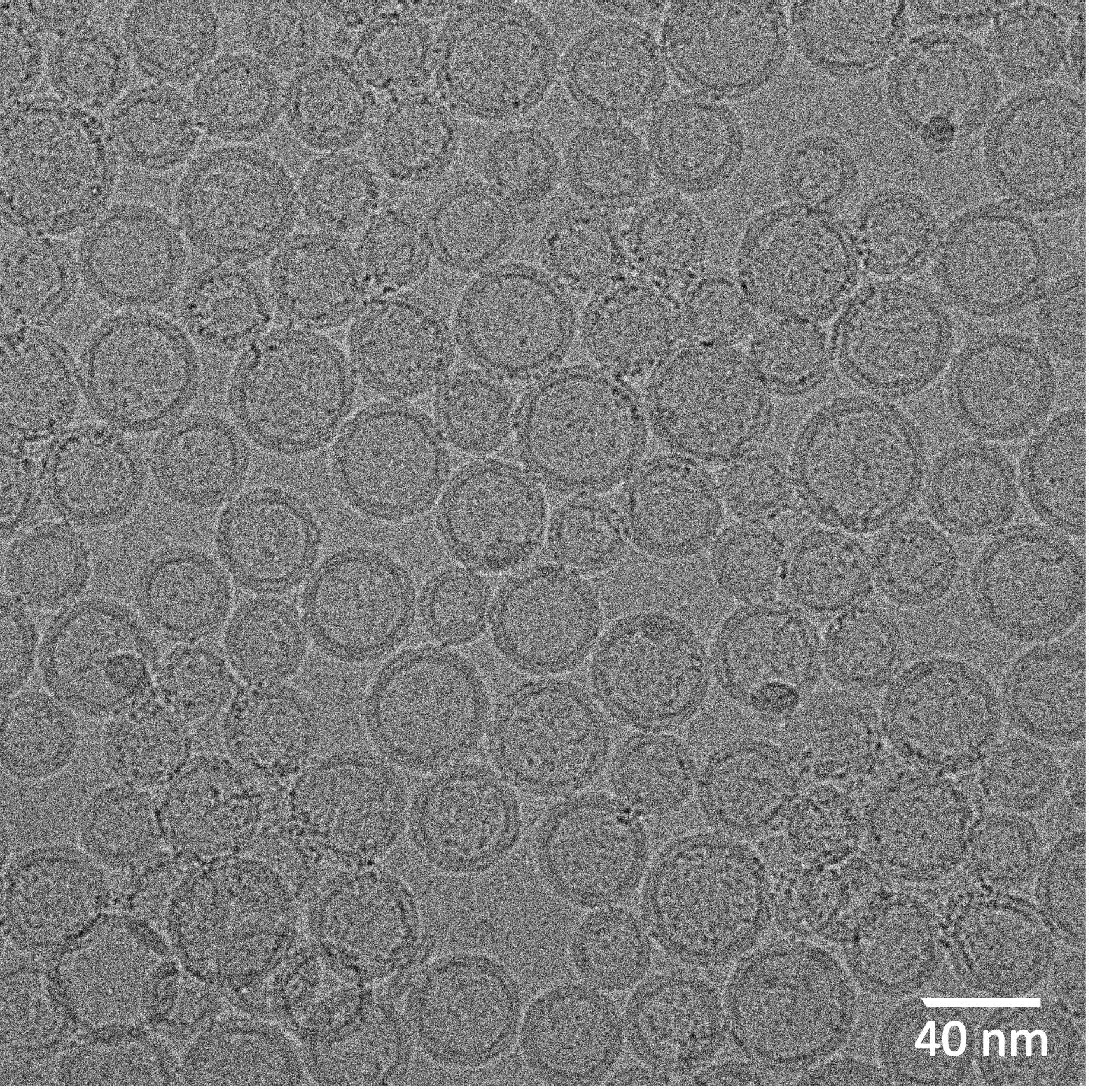
Cryo-EM of POPC vesicles formed by dilution of 0.5 q-value POPC/DHPC bicelle mix.
Background
The fundamental building blocks for biomembranes are phosphoglycerides. Their low solubility and amphiphilic nature enable these lipids to self-assemble into various structural mesophases including bilayers, the basic barriers that compartmentalize cells. Cells take advantage of membrane bilayers to form internal and external barriers, as well as to carry out transport of materials within and between cells. The latter process involves generation of bilayer vesicles to act as delivery shuttles, by budding from and fusing with different membranes. Such functionality is critically important during periods of cell growth, development, and proliferation for recycling and re-utilization of biomolecules during programmed cell death processes, and for neurotransmission. To gain insights into the physicochemical properties that enable cell membrane functionality, studies of model bilayer membranes produced in vitro, i.e., liposomes, have proven invaluable. The studies have also led to biotechnological advances that include the engineering of liposomes for delivery of drugs, genes, and vaccines, as well as for applications in medical diagnostics, cosmetics, and food industry-related nutraceutics and dietetics (Lichtenberg and Barenholz, 1988; Laouini et al., 2012; Akbarzadeh et al., 2013; Keller et al., 2013; Patil and Jadha, 2014; Nkanga et al., 2019; Maja et al., 2020; Ajeeshkumar et al., 2021).
Over the years, a variety of methodologies have been used to generate bilayer liposomes.Generally, approaches that result in homogeneous populations of single-wall, i.e. unilamellar, bilayer vesicles are preferred to mimic the situation generally encountered in cells, and to optimize trapping efficiency using the least amount of lipid. In biomedical research, approaches involving ultrasonication, reverse-phase evaporation, and extrusion have been widely used. Less widely used are ethanol injection, detergent dialysis/removal, and supercritical fluid approaches. Nearly all of the approaches are either tedious, time-consuming, require specialized equipment, and produce vesicles with limited shelf-life at either room temperature or in cold storage.
We sought a straightforward approach for fast and easy construction of homogeneous unilamellar bilayer vesicles comprised of 1-palmitoyl-2-oleoyl phosphatidylcholine (POPC). We turned to bicelle-based approaches because of insights provided by theoretical and molecular dynamics modeling of mechanistic pathways, involving bicelle disc transformation into unilamellar bilayer vesicles (Fromherz, 1983; Marrink and Mark, 2003; Chng, 2013). These studies suggest that loss of rim-stabilizing short-chain PC (e.g., dihexanoyl PC; DHPC) or detergents from bicelles will lead to their coalescence into larger-diameter discs, followed by the formation of cup-shaped intermediates to reduce long-chain PC hydrocarbon chain exposure to water. With further departure of short-chain DHPC or detergent from the rim areas, the cup-shaped hemi-vesicle intermediates are predicted to undergo rim closure to form unilamellar vesicles. From a thermodynamic standpoint, the loss of DHPC or detergent from the bicelle rims alters the energetic balance between the rim perimeter line tension and the bicelle disc elastic bending energy, with the perimeter line tension becoming more dominant, as DHPC or detergent is lost from the bicelle rim. This situation promotes formation of the cup-shaped hemi-vesicle intermediates that further transition to unilamellar vesicles when nearly complete loss of rim-stabilizing DHPC occurs.
In the majority of previous experimental studies involving bicelles, the long-chain phosphoglyceride component contains saturated acyl chains, e.g., dimyristoyl phosphatidylcholine (DMPC) or DMPC/dimyristoyl phosphatidylglycerol mixtures (DMPG). These lipid species are generally absent or very minor components of eukaryotic biomembranes. Typically, biomembrane phosphoglyceride acyl composition is asymmetric, with the sn1-chain being long and saturated (C ≥ 16), while the sn2-chain is long (C ≥ 18) and contains cis unsaturation, as occurs in POPC (Figure 1). This arrangement has interesting biological consequences, including more efficient membrane vesiculation, and lower membrane permeability compared to phosphoglycerides containing two saturated or polyunsaturated chains (Manni et al., 2018).
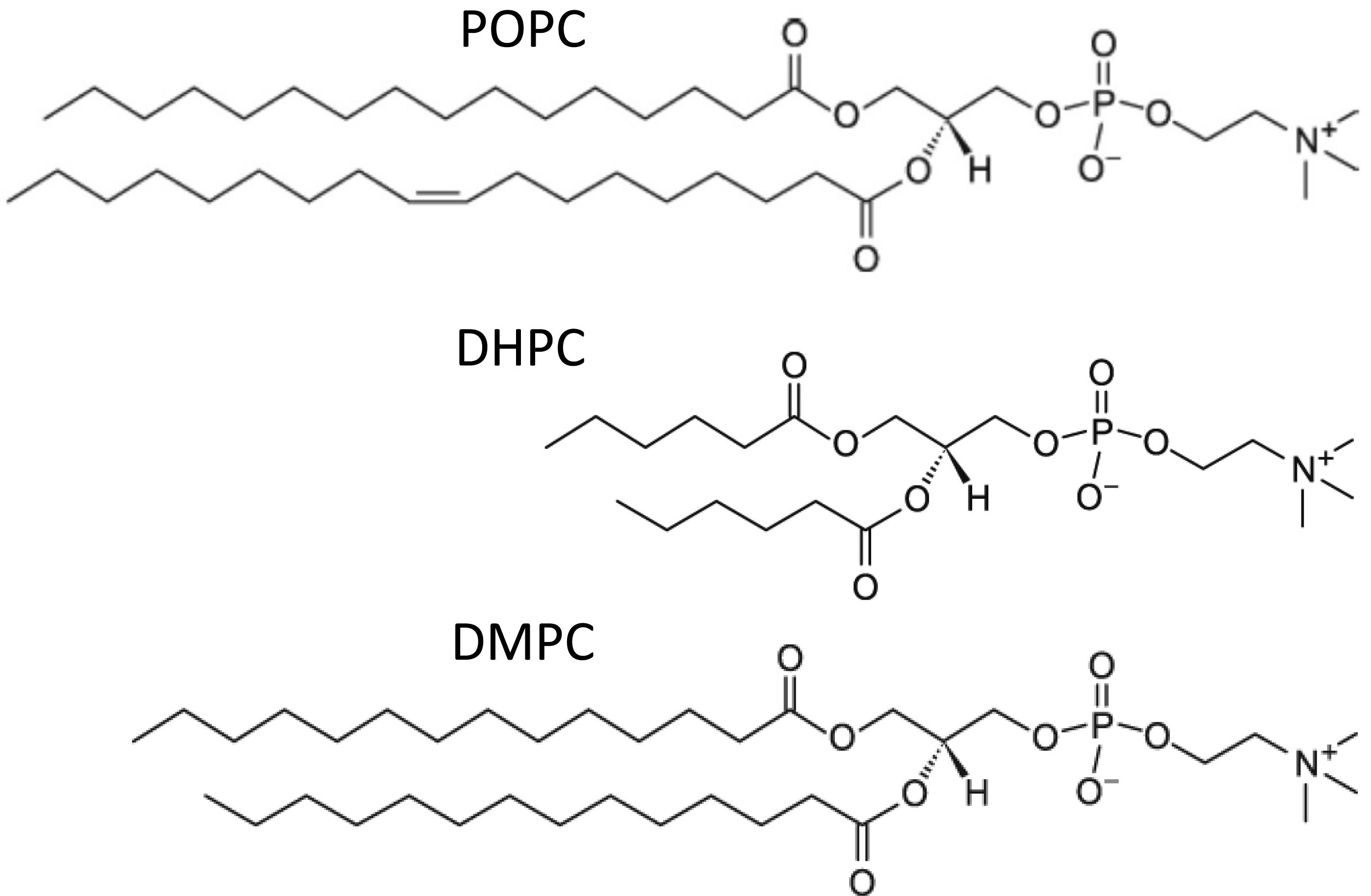
Figure 1. Chemical structures of phosphatidylcholines used or discussed in this study.
From previous studies, it is known that the structural stability of DMPC/DHPC bicelles is critically dependent upon the total concentration of long-chain PC+DHPC/detergent (Sanders and Schwonek, 1992; Sanders and Prosser, 1998; Harroun et al., 2005; Lu et al., 2012; Beaugrand et al., 2014). Dilution of bicelles after formation can alter the long-chain PC-to-DHPC/detergent ratio (q-value) and drive mesomorphic structural change, including transformation to liposome bilayer vesicles (Nieh et al., 2004, 2009 and 2011; Yue et al., 2005; Mahabir et al., 2013). The dilution-driven structural transformation is a consequence of the vastly differing aqueous solubilities of the long-chain PC versus the DHPC/detergent lipids forming the bicelle. In the case of DMPC/DHPC mixtures, the aqueous solubilities of DMPC (~6 nM) and DHPC (~16 mM) differ by 2.7 million-fold. Thus, when the bicelle concentration is suddenly decreased and becomes too low (as can occur with aqueous dilution), DHPC moves from the bicelle into aqueous phase. This situation changes the q-value and alters the structural form of the bicelle lipid mixture. This response is accounted for by q*, through provision of the effective DMPC/DHPC molar ratio for bicelle mixtures at various lipid concentrations (Beaugrand et al., 2014). For example, dilution of 1.0 q-value DMPC/DHPC mixtures from 75 to 25 mM results in q increasing from 1.0 up to a q* of 1.8, where isotropic bicelles still prevail. Upon dilution to 16 mM total PC, a 3.2 q*-value results in the prevalence of large bicelles. Further dilution to 2 mM total PC leads to an extremely high q*, where small DMPC liposome vesicles become detectable by 31P-NMR.
From a practical standpoint, whether the preceding modeled and experimental behavior that characterizes DMPC/DHPC and DMPC/DMPG/DHPC bicelle lipid mixtures can yield a simple and easy method for producing homogeneous populations of POPC unilamellar bilayer vesicles has remained unclear. Recently, a straightforward approach that streamlines production of POPC vesicles from POPC/DHPC bicelle mixtures has been used in applications for monitoring protein-mediated intervesicular lipid transfer activity (Gao et al., 2020). Characterization has revealed these POPC vesicles to be small (32-36 nm diameter), unilamellar, bilayer vesicles that are surprisingly homogeneous, stable, resistant to freeze-thaw alteration, and usable in assays involving cell cytosolic fractions. The pathway for vesicle formation is illustrated in Figure 2 and production details follow:
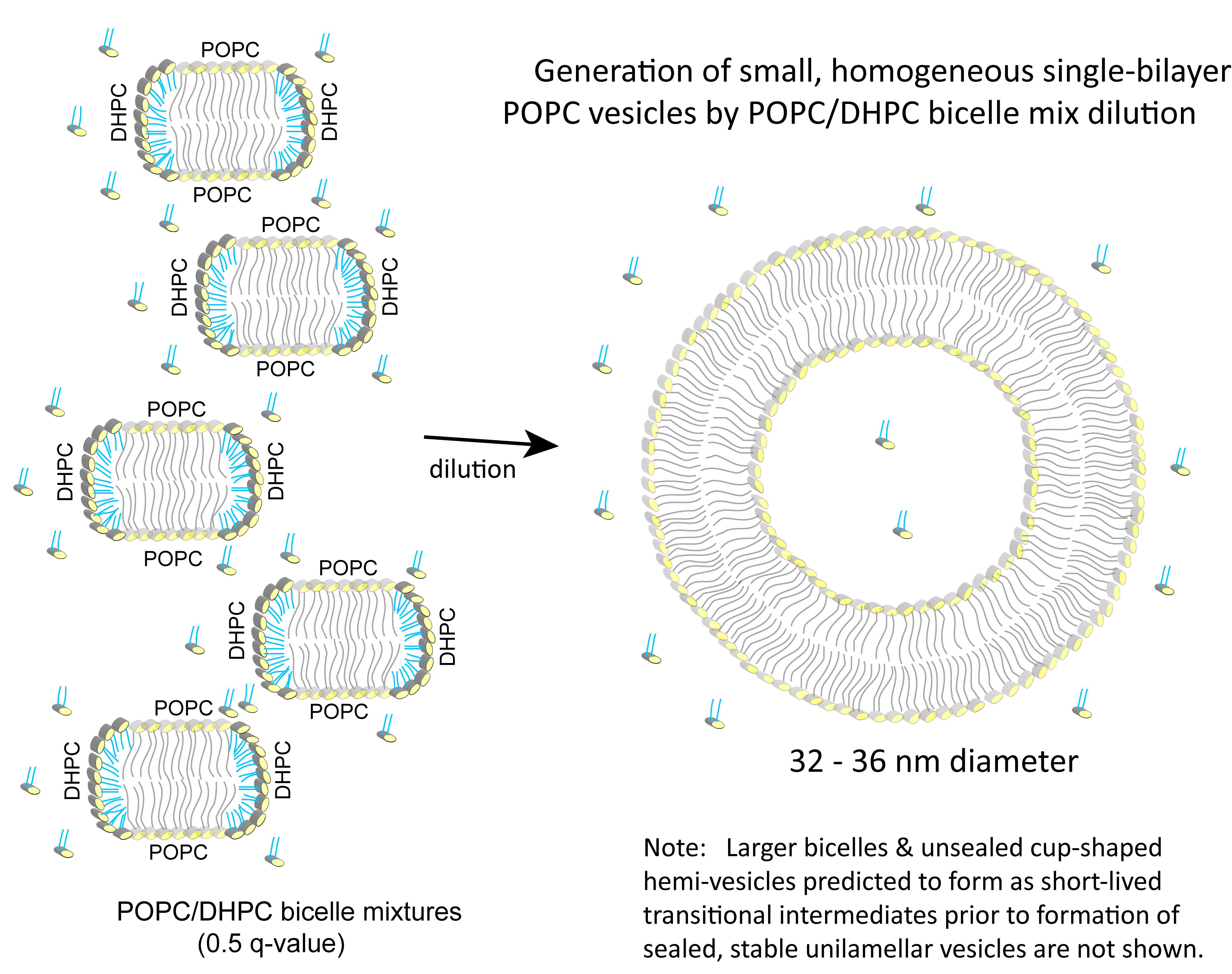
Figure 2. Generation of small, homogeneous single-bilayer POPC vesicles by dilution of POPC/DHPC bicelle mixtures. The phosphorylcholine polar head groups are shown as yellow ellipsoids with the long acyl chains of POPC depicted in gray (stick fashion) and the short acyl chains of DHPC depicted in cyan (stick fashion). As noted, dilution (>125-fold) of the 0.5 q-value POPC/DHPC bicelle mixture with aqueous buffer initially leads to larger bicelle formation (not shown), followed by transition to unsealed cup-shaped hemi-vesicles (not shown), that seal to form stable unilamellar vesicles. Illustrations of these short-lived transitional intermediates are depicted in Fromherz (1983), Chng (2013), or Marrink and Mark (2003). The bicelles (left) and vesicle (right) are shown in two-dimensional cross-section.
Materials and Reagents
Disposable Pasteur pipets (5.75” borosilicate glass, nonsterile; Fisherbrand, catalog number: 13-678-20A)
Kimble clear glass screw-thread sample vials with PTFE closure (Fisher, catalog numbers: 03-340-60A [4 ml, 17 × 60 mm]; 03-340-60C [12 ml, 19 × 65 mm])
MininertTM syringe valves, leak-tight PTFE closures for screw cap vials and valve open/close action enabling Hamilton syringe access ensures that only the PTFE and the glass come in contact with the lipid sample solution dissolved in nonpolar solvent mixes [e.g., chloroform/methanol (2:1) or hexane/isopropanol/water (7:3:0.25)]
Conical V-bottom vials (clear glass, Reacti-VialTM 1 ml capacity; Thermo Scientific, catalog number: 13221)
Glass syringes, gastight, assorted volumes (Hamilton, catalog numbers:1702 [25 µl]; 1705 [50 µl]; 1710 [100 µl]; 1725 [250 µl]; 1750 [500 µl]; 1001 [10,00 µl])
1-palmitoyl-2-oleoyl phosphatidylcholine (POPC) (Avanti Polar Lipids, Croda Intl., catalog number: 850457)
Dihexanoyl phosphatidylcholine (DHPC) (Avanti Polar Lipids, Croda Intl., catalog number: 850305)
Hexane, HPLC grade (Fisher Chemical, catalog number: H302-4)
Isopropanol, HPLC grade (Fisher Chemical, catalog number: A451-4)
Chloroform, stabilized with 0.75% EtOH (Mallinckrodt, catalog number: 4440-08)
Methanol (Fisher Chemical, catalog number: A412-4)
Nitrogen gas
Cahn style aluminum microbalance weigh pans (Mfr. # 120627) (Thomas Scientific, catalog number: 1162D64)
Equipment
100 ml beaker
Nitrogen tank equipped with gas pressure regulator
Lab support stand (8.3” × 5.5”) with rod (19.7”), lab stand clamp holder, and 3-prong finger-style clamp for holding disposal glass Pasteur pipette linked to N2 tank with Tygon tubing, for evaporating organic solvent from lipid sample (< 2 ml) using N2 gas in lab exhaust hood.
Note: N-EVAPTM Analytical Evaporator (Organomation, Model 111 or 112 24 slot system) is useful when simultaneous evaporation of organic solvents from several small volume (< 2 ml) lipid samples using N2 gas is needed.
Lab chemical fume exhaust hood, e.g., Supreme Air (Keewaunee Sci. Corp.)
Rotary evaporator (Buchi R-300 Rotavapor model 11R300152V002) (Fisher Scientific, catalog number: 05-000-485) [needed if lipids are dissolved in large solvent volumes (e.g., >5-10 ml)]
Duo-Seal Vacuum Pump (Welch, model: 1400B-01); generates vacuum of ~0.001 Torr w/ pumping speed of 25 L/min
Vortex Genie 2 (Daigger Scientific)
Bath Sonicator (Branson, Model 2200 or Model 22-4)
KIMAX glass vacuum dessicator, 15 cm plate size (Cole Parmer, catalog number: EW-06536-11)
Ohaus Analytical Plus Microbalance (Precision Weighing Balances, Model AP250D)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Gao, Y. G., My Le, L. T., Alam, A. and Brown, R. E. (2021). A Simple and Straightforward Approach for Generating Small, Stable, Homogeneous, Unilamellar 1-Palmitoyl 2-Oleoyl Phosphatidylcholine (POPC) Bilayer Vesicles. Bio-protocol 11(24): e4271. DOI: 10.21769/BioProtoc.4271.
分类
生物化学 > 脂质 > 膜脂
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link