- EN - English
- CN - 中文
Single Molecular Resolution to Monitor DNA Replication Fork Dynamics upon Stress by DNA Fiber Assay
通过 DNA 纤维测定法监测 DNA 复制叉动力学的单分子分辨率
发布: 2021年12月20日第11卷第24期 DOI: 10.21769/BioProtoc.4269 浏览次数: 5259
评审: Alice MeroniArif Md. Rashedul KabirAnonymous reviewer(s)
Abstract
DNA replication always encounters numerous endogenous and exogenous stresses during the cell cycle. Measuring the cell responses to stress has primarily relied on cell survival and incorporation of radioactive dNTPs, which is limited in resolution. A higher resolution is required to monitor how replication and repair respond to these stresses. This protocol describes a procedure to monitor the length of new synthesized DNA in a single molecular resolution called DNA fiber assay. The fiber assay relies on labeling of nascent DNA with the nucleoside analog 5-Chloro-2'-deoxyuridine (CldU) and 5-Iodo-2'-deoxyuridine (IdU). We can visualize the labeled nascent DNA in single molecular resolution by immunostaining. By measuring labeled DNA length, the assay permits interrogation of replication speed at given conditions, end processing at the reversed fork, and fork restart after repair.
Background
The fidelity of DNA replication is always challenged by exogenous and endogenous sources of stress, including base lesions and crosslinks generated from genotoxic reagents, secondary DNA structures generated from GC enriched regions, and transcription interference (Berti et al., 2020a). Upon replication stress, the DNA polymerase uncouples from CMG helicases and the replication speed will slow down to allow the repair of the lesions. Meanwhile, the uncoupling leads to more ssDNA at the stalled fork (Zellweger et al., 2015). Fork reversal is the global response to replication stress, which indicates new synthesized leading and lagging strands annealed together by DNA translocases and RAD51. The reversed fork is protected by many protection factors to prevent degradation mediated by nucleases (Schlacher et al., 2011 and 2012; Mukherjee et al., 2019; Liu et al., 2020). The intact reversed fork confers the stability of the stalled fork, facilitating later repair pathways, such as template switch to restart the fork (Bhat and Cortez, 2018). Moreover, Primpol mediated repriming and translesion synthesis are also involved in the replication stress response (Bai et al., 2020; Quinet et al., 2020; Genois et al., 2021).
To understand how the fork is protected and repaired upon stress, we need to monitor fork activities such as fork speed, ssDNA gaps, fork reversal, nascent DNA strand degradation (NSD), and fork restart at a single molecular resolution. We and others have previously shown that the DNA fiber assay can successfully monitor fork activities in different cell lines at a single molecular resolution (Liu et al., 2016 and 2020; Dungrawala et al., 2017; Bai et al., 2020; Quinet et al., 2020; Genois et al., 2021). We cultured cells for a fixed time with the warm complete medium containing a high concentration of thymidine analogs CldU or IdU. The thymidine analogs integrated in the genome DNA after a certain treatment, the cells were harvested and lysed on slides, followed by DNA fixation. The DNA is denatured and then the labeled DNA undergoes immunostaining. Two monoclonal BrdU antibodies generated from mouse and rat are able to cross-react with IdU and CldU, respectively, so we took the advantage of this cross-reaction to immunostain the analogs with distinct colors. To be consistent, in this paper, we stain CldU with red and IdU with green. We could image the labeled single DNA molecule and quantify fork activities by fluorescence microscopy scanning.
Thus far, the DNA fiber assay was used to determine fork speed (Mehta et al., 2020; Krishnamoorthy et al., 2021), fork reversal in some fork protection deficiency cells (Mijic et al., 2017; Taglialatela et al., 2017; Krishnamoorthy et al., 2021) such as the BRCA2 null cell line, nascent DNA strand degradation (Dungrawala et al., 2017; Przetocka et al., 2018; Liu et al., 2020), ssDNA gaps after Primpol mediated repriming (Bai et al., 2020; Quinet et al., 2020; Genois et al., 2021), fork restart in various treatments, and genetic background (Liu et al., 2016; Palermo et al., 2016; Mason et al., 2019).
To monitor different fork activities, we used different labeling strategies and treatments (Figure 1A for labeling, see notes for analysis). For measuring fork speed in U2OS cells, we labelled cells with CldU for 20 min, followed by IdU for 20 min. We measured the CldU and IdU labeled DNA lengths and plotted their length separately. Longer fibers meant faster replication speed, or DNA synthesis. Interestingly, we could also see fibers with one color in the middle of another color. When a green fiber has a red color in the middle, this indicated the formation of a new firing origin (Figure 1B), and when a red fiber has green in the middle, this indicated a termination event (Figure 1C). By plotting the length ratio of longer green fibers to shorter green fibers in an origin firing, we may determine if the stress or genetic background causes fork progression problems (Dungrawala et al., 2017). In a previous study, the fork speed labeling method with low dose CPT (50nM) was also used to monitor fork reversal frequency (Berti et al., 2020b), since fork reversal correlates with fork speed in some specific background, such as low dose CPT. The cells were firstly labeled with CldU for 20 min, followed by IdU labeling for another 20 min, and co-treated with low-dose CPT during IdU labeling (Figure 1A). The measurement for fork reversal was the ratio of IdU/CldU, with shorter IdU fiber length corresponding to more fork reversal.
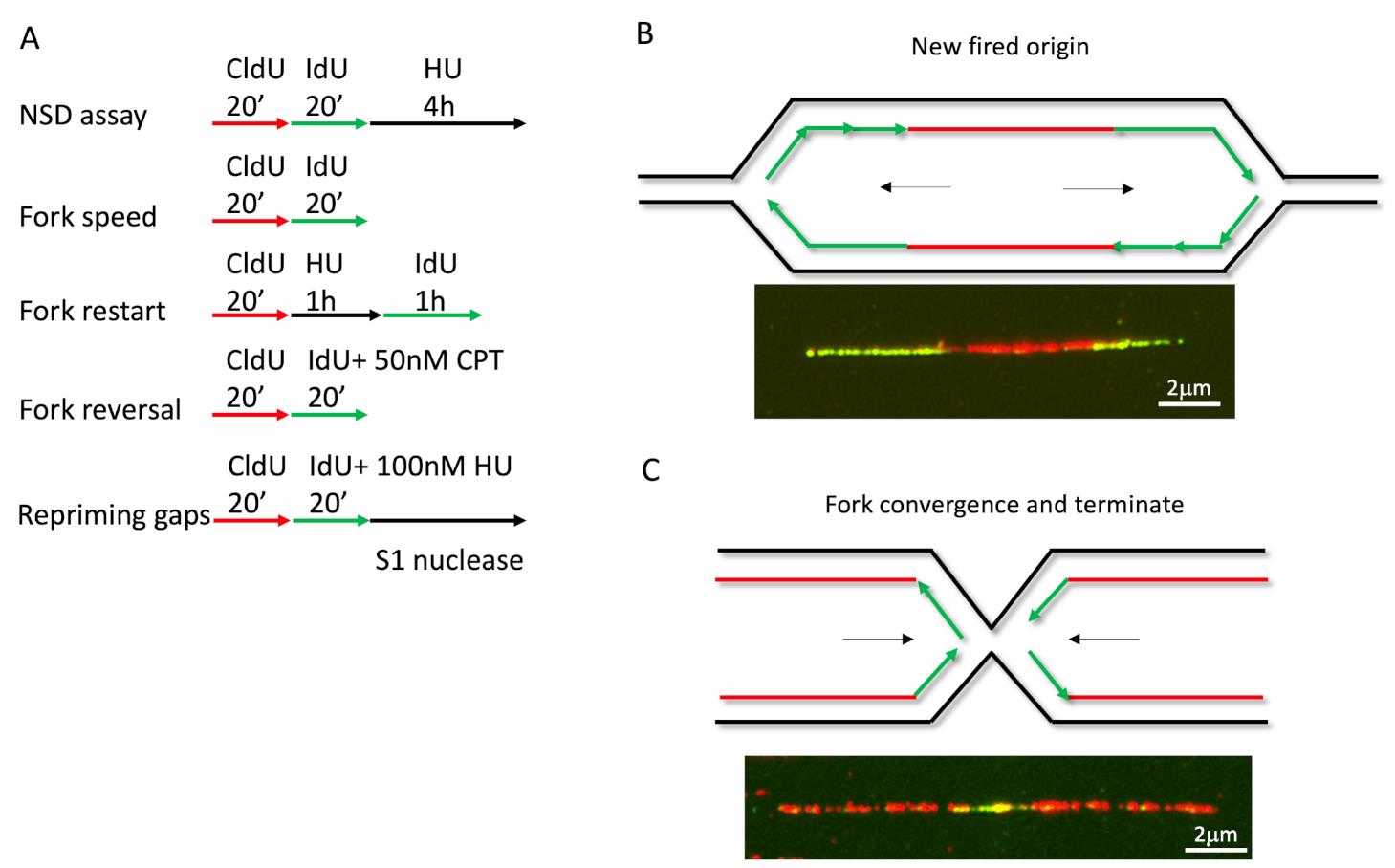
Figure 1. DNA fiber labeling in different applications. (A) Cells labeled with CldU and IdU in different protocols to determine various fork activities. (B-C) Examples for new firing origins and terminations events.
Loss of fork reversal enzyme HLTF leads to repriming dependent aberrant fork speeding, but leaves ssDNA gaps behind (Bai et al., 2020). To detect ssDNA gaps generated by the repriming pathway, the labeling method is shown at the bottom of Figure 1A. After pulsing with thymidine analogs, cells are harvested and digested with S1 nuclease before spreading. The S1 nuclease is a single-strand-specific endonuclease that cleaves ssDNA at the dsDNA region caused by a nick, gap, mismatch, or loop. S1 nuclease treatment digests the IdU labeled fibers with gaps into short pieces that are undetectable; as a consequence, IdU fibers become shorter than CldU fibers.
Another application for this method is the nascent DNA strand degradation assay. Briefly, cells treated for equal duration with CldU and IdU, followed by 5 h high dose Hydroxyurea (4 mM) treatment, can fully stall replication (Figure 2A). The fiber length of CldU and IdU is noted, and the ratio of IdU/CldU is plotted as the degradation readout. Theoretically, if the IdU/CldU ratio equals 1, the fork remains intact (Figures 2B and 2C), but if the ratio is less than 1, this indicates the nascent DNA has been degraded by nucleases (Figure 2D). The nascent DNA strand degradation can be rescued by either blocking nucleolytic digestion or inactivating fork reversal (Figures 2B and 2C). Previous studies show that fork reversal is the entry point for NSD. Therefore, an NSD assay is also used to measure fork reversal in some specific situations (Liu et al., 2020).
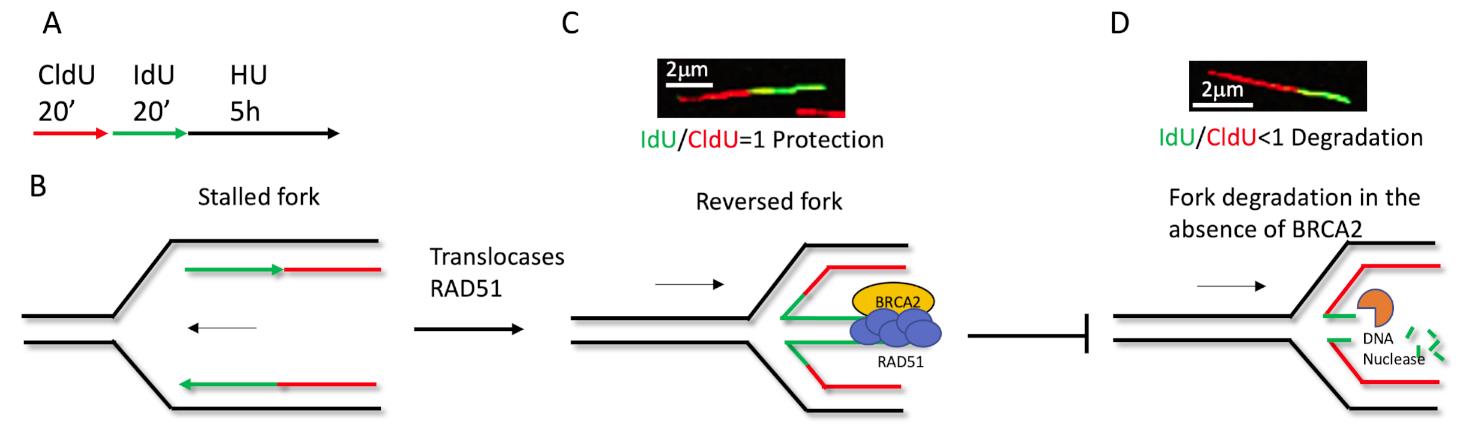
Figure 2. Schematic of how the fiber labeling technique detects nascent DNA degradation in the stressed fork. (A) Cells labeled with 20 μM CldU and 100 μM IdU for 20 min, respectively, followed by 4mM HU for 5 h. (B) The replication fork is stalled upon HU treatment, after CldU and IdU labeling. Then, RAD51 and translocases mediate fork reversal (C), BRCA2 stabilizes RAD51 at the regressed arm to protect the fork from nascent DNA degradation by nucleases (D). In the absence of protection, such as BRCA2 deficiency, the newer synthesized DNA will degrade first, causing the IdU/CldU ratio to be less than 1. Either blocking the nucleolytic digestion, restoring protection, or inactivating fork reversal can prevent degradation, an increase the ratio of IdU/CldU back to 1.
To monitor fork restart efficiency, we labelled the cells with CldU for 20 min, followed by 1 h high dose HU or other reagents of interest. After wash out of the stress reagents, 20 min to 1 h of IdU without stress allowed replication restart. Through imaging, we can see fibers with green connecting red, which means a restart event. Red only fibers indicate stalled forks that failed to restart. Green only fibers indicate new firing origins. The measurement of restart is calculated by counting the number of dual-color fibers and red-only fibers. We plot the restart efficiency by the ratio of Nred/green/(NRed only + Nred/green). There are fibers that have red in the middle of green or green in the middle of red in the fiber image. These fibers indicate the fork restart first, followed by termination events. However, it is hard to know if these fibers indicate one or two restart events, since we usually count it as one restart event.
In this protocol, we use the NSD assay as an example to describe the labeling, staining, and data analysis. We knockdown BRCA2 as an example to test NSD, because this gene is required in fork protection (Schlacher et al., 2012).
Materials and Reagents
Premium Microscope Slides Frosted (Fisherfinest, catalog number: 12-544-2)
Corning 24 × 50 mm Rectangular Cover Glass (Fisher Scientific, catalog number: 12-553-464)
6-well plate (Corning, catalog number: 07-200-83)
Plastic bags (Uline, catalog number: S-952)
U2OS cells (ATCC)
DMEM (Gibco, catalog number: 11965-092)
FBS (Gibco, catalog number: A4766801)
Rat monoclonal anti-BrdU (Abcam, catalog number: ab6326, store at -20°C)
Mouse anti-BrdU (Becton Dickinson, catalog number: 347580 B44, store at 4°C)
Goat anti Rat IgG (H+L) Secondary Antibody, Alexa Fluor 594 (Invitrogen, catalog number: A-11007, 4°C, avoid light)
Goat anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Invitrogen, catalog number: A32723, 4°C, avoid light)
Prolong Gold without DAPI (Invitrogen, catalog number: P36930, RT)
HBSS (Gibco, catalog number: 14175-095)
BRCA2 siRNA (Dharmacon, catalog number: L-003462-00-0005, dissolved in nucleases free water at 20 µM)
Non-targeting siRNA (siNT Qiagen, catalog number: 1027281)
Dharmafect 1 (Dharmacon, catalog number: T-2001, store at 4°C)
Opti-MEM (Gibco, catalog number: 31985-070)
EDTA 0.5M pH 8.0 (Corning, catalog number: 46-034-CI, RT)
SDS 10% (Sigma, catalog number: 75746-250G; dissolve in water, store at room temperature for 1 year)
Tris-HCl pH7.4 1 M (Sigma, catalog number: 77-86-1; dissolve in water, adjust pH to 7.4 with HCl, store at room temperature for 1 year)
Triton X-100 (Sigma, catalog number: T8787)
Glacial Acetic acid (Fisher , catalog number: A38-212)
Methanol (Fisher Scientific, catalog number: A413-20)
Hydrochloric Acid (Fisher Scientific, catalog number: A144-212)
Goat serum (Gibco, catalog number: 16210072, aliquot to 1ml eppendoff tubes in -20°C)
Trypsin (Gibco, catalog number: 25200056)
IdU (Sigma, catalog number: I7125, powder in -20°C, resuspended in 1 N ammonium hydroxide at 50 mg/ml or 141.2 mM; aliquot to 20 μl, store in -20°C for up to 3 years) (see Recipes)
CldU (Sigma, catalog number: C6891, powder in -20°C, resuspended in 1 N ammonium hydroxide at 20 mg/ml or 76.1 mM, store at -20°C for up to 3 years) (see Recipes)
HydroxyUrea (HU; Sigma, catalog number: H8627) (see Recipes)
Fiber spreading buffer (see Recipes)
Denature buffer (see Recipes)
Blocking buffer (see Recipes)
Equipment
Spreading apparatus (see Figure 3 how to make it)
Immunofloursence microscope (Nikon, model: Ti-DH)
Coplin jar
Incubator or water bath
Software
Nikon Nis elements BR 4.40.00 64-bit
Prism 8
Excel
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Liu, W. (2021). Single Molecular Resolution to Monitor DNA Replication Fork Dynamics upon Stress by DNA Fiber Assay. Bio-protocol 11(24): e4269. DOI: 10.21769/BioProtoc.4269.
- Liu, W., Krishnamoorthy, A., Zhao, R. and Cortez, D. (2020). Two replication fork remodeling pathways generate nuclease substrates for distinct fork protection factors. Sci Adv 6(46): eabc3598.
分类
生物物理学 > 单分子技术
生物物理学 > 显微技术
生物化学 > DNA > 单分子活性 > 成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













