- EN - English
- CN - 中文
Assessment of Corticospinal Excitability in Awake Rodents Using EMG-Controlled Intracortical Stimulation
使用 EMG 控制的皮质内刺激评估清醒啮齿动物的皮质脊髓的兴奋性
发布: 2021年12月20日第11卷第24期 DOI: 10.21769/BioProtoc.4267 浏览次数: 2693
评审: Geoffrey C. Y. LauStefania Bruni
Abstract
Assessment of corticospinal excitability (CSE) is an essential component of experiments designed to induce or study neuronal plasticity in the motor system. Common examples are paired associative stimulation (PAS), theta-burst stimulation (TBS), intensive motor training, or any methods aimed at potentiating the corticomotor system in the hope of promoting better recovery after neurological insult. To date, rodent models of CSE assessment have mostly been completed under anaesthesia, which greatly affects the level of CSE, as well as the mechanisms of plasticity. Experiments in awake animals are difficult because the ongoing state of behavior affects the excitability of the motor system and complicates the assessment of CSE. To address this issue, we have designed a novel approach for CSE assessment in awake behaving rodents, enabling a reliable measure of evoked motor responses obtained from cortical microstimulation in repeatable conditions of ongoing motor activity. The system relies on chronically implanted intracortical and intramuscular electrodes and a custom-made software control system, enabling the user to require that precise parameters of EMG activity be met before cortical stimulation probes are delivered. This approach could be used for further studies of PAS, TBS or other interventions requiring the assessment of CSE under repeatable conditions. We provide fabrication schematics and a list of materials for the implant, as well as instructions for running a custom-made MATLAB codebase, customizing the PAS protocol, and performing the complete analysis of experimental data. We hope these tools can further facilitate animal research in the field of neuroplasticity and neurorehabilitation.
Background
Corticospinal Excitability
Neuroplasticity and sensorimotor network reorganization are determining factors for functional recovery after stroke (Dancause and Nudo, 2011; Zeiler and Krakauer, 2013) and spinal cord injury (Serradj et al., 2017; Brown and Martinez, 2019). The corticospinal system is the major neural pathway facilitating fine voluntary motor control in non-human primates and humans, connecting the major output neurons in layer Vb of the motor cortex with the spinal circuits controlling arm and hand muscles (Lemon, 2008). A deeper understanding of how beneficial plasticity can be induced in this pathway will be instrumental to designing better therapeutic interventions after stroke. Research in humans and non-human primates can directly investigate the fine motor function of hands and digits. Despite the fact that rats lack monosynaptic corticomotoneuronal cells (Alstermark et al., 2004) and opposable thumbs or individuated finger movement, they have a good level of distal forelimb dexterity and constitute a useful model of forelimb motor function and recovery from lesion (Kleim et al., 2007). They also present significant advantages for research over non-human primates in terms of ethical justification, cost, and the growing availability of transgenic strains. A potential difficulty in using rodent models to investigate corticospinal plasticity resides in obtaining accurate and consistent measures of corticomotor pathway excitability during behaviour, without the influence of anaesthesia. We have approached that goal by implementing a novel EMG-controlled intracortical micro-stimulation approach using chronically implanted electrodes in the rat. The purpose of this paper is to further document and make available the blueprints for our system as a companion to the main scientific paper (Ting et al., 2020), so that the interested parties may use and adapt them to suit their needs.
Paired Associative Stimulation (PAS) to Modulate Corticospinal Excitability
One demonstrated method to modulate corticospinal excitability (CSE) is paired associative stimulation (PAS). In humans, transcranial magnetic stimulation and transcutaneous neuromuscular stimulation are non-invasive stimulation methods used to target the motor cortex and a contralateral peripheral nerve. The concept, based on Hebbian spike-timing-dependent plasticity (Dan and Poo, 2004; Feldman, 2012), is that repetitive forced coincidence of pre- and post-synaptic action potentials can lead to a LTP-like conditioning of the intervening synapses, and result in a beneficial strengthening of corticomotor connections. The first PAS studies aimed to induce plasticity in the sensorimotor cortex (Stefan et al., 2000), but subsequent investigations confirmed the possibility for spinal plasticity as well (Taylor and Martin, 2009). In the latter case, antidromic potentials in motoneurons were made to coincide with orthodromic descending corticospinal volleys to transiently modulate CSE.
PAS has been shown to increase corticomotor excitability in healthy human participants (Suppa et al., 2017) and promote recovery after human neurological insult (Rothwell, 2016; Urbin et al., 2017; Christiansen and Perez, 2018). However, PAS effects are highly variable across subjects (Sale et al., 2007; McGie et al., 2014; Tarri et al., 2018), and failure at inducing plasticity with PAS has also been reported (McGie et al., 2014). Therefore, the promise of neuromodulation therapies such as paired stimulation warrant further studies in animal models (Mishra et al., 2017), to improve our understanding of the factors leading to effective control of neuronal plasticity for functional benefits.
There are sparse animal investigations in the PAS literature. Most published studies are completed under anesthesia, or require some degree of restraint (Zhang et al., 2018). Hence there continues to be an unaddressed need to develop a readily extensible chronic model of CSE in awake, ecologically behaving rats. Such a system should provide a way of assessing the CSE during normal behavior, while ensuring that the CSE assessments are made at similar levels of corticomotor activity. It should also permit an effective delivery of therapeutic stimulation protocols. Here, we present a closed-loop CSE assessment model, which provides a reliable means to investigate the effectiveness of interventions aimed at driving corticospinal plasticity such as PAS. This closed-loop, EMG-controlled CSE assessment protocol contains a list of equipment and fabrication instructions. We also include detailed instructions on a functional MATLAB codebase that we developed specifically to acquire and analyze data obtained from PAS experiments using this model. In this way, we demonstrate a full prototyping ecosystem for conducting closed-loop CSE assessment in rodents.
We first discuss the specific goals of our system along with the roles of each major component, and follow with the main protocol describing the implant surgery. Standalone sub-protocols are also included, detailing step-by-step fabrication of (1) the cortical array to be inserted in the brain; (2) the cortical connector to which the cortical array attaches, including the EMG wires to be inserted in the muscle; and (3) the connector cable which tethers the rat to (4) the hardware and software platform which permits closed-loop EMG recording and control. Explanatory pictures and schematics are provided at key steps to aid understanding and help facilitate assembly of the necessary components.
General Approach
In designing this system, we had several considerations in mind:
A chronic preparation to preclude the need for anesthesia. Ketamine-xylazine and other associated agents have an effect on NMDA-dependent plasticity, due to their purported mechanism as NMDA-receptor antagonists (Zanos and Gould, 2018). We wanted to streamline the process so that only one recovery surgery is required to perform the full implant, for ethical concerns as well as for efficiency.
The system should permit repeated assessment of CSE under relatively consistent conditions of EMG activity. We selected the trapezius, biceps and extensor carpi radialis (ECR) as three potential plasticity targets and for EMG readout, because electrical and excitatory optogenetic stimulation of the caudal forelimb area (CFA) in the primary motor cortex of the rat is most likely to induce movements in one or more of these muscles. The ECR was the primary target because it is involved in fine motor control of the wrists, forepaws and fingers of the rat, and one goal of this model is to facilitate the study of dexterity and neurostimulation after motor cortex lesioning.
CSE assessment or any interventions should not require fixed head or limb restraint, but perhaps a temporary tether permitting a high degree of movement flexibility.
We wanted to have some degree of redundancy in the cortical and muscle stimulation. In case one or more electrodes failed, we could still continue with a PAS intervention and assessment of CSE with a different target muscle. This could reduce the total amount of rats required for a particular study.
Our overall design is shown in Figure 1, with details in Figure 2. The implant itself consists of three main components: (1) a 2 × 2 array of electrodes in the primary motor cortex (Figure 3), which is used to stimulate the descending corticomotor tract, thus inducing a motor evoked potential (MEP) which could be measured by the (2) EMG electrodes embedded in the wrist extensor, shoulder and biceps muscles, and (3) a Samtec® head-mounted connector with clipping latches, which permitted detachable and reliable interfacing with a nearby computer.
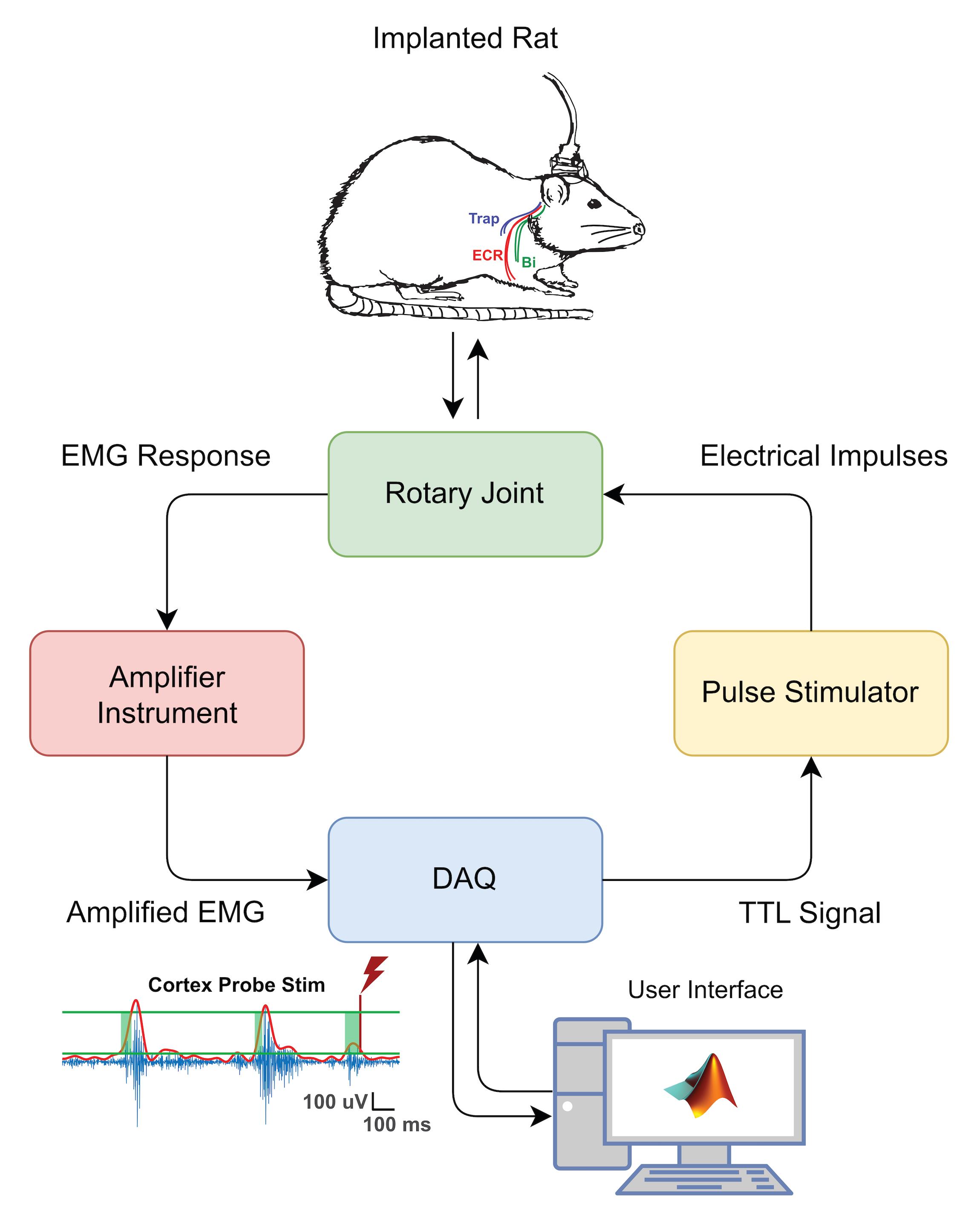
Figure 1. CSE Assessment Hardware Schematic. Stimulation protocols and EMG data capture are facilitated by a Data AcQuisition (DAQ) interface on a desktop computer. The implanted rat (top) has both cortical and EMG electrodes, and both sets are connected to an access port on the rat’s head. A custom-made cable connects this access port to a fixed frame with a rotary joint commutator, permitting relatively free movement and preventing significant torsion on the cable. The raw EMG signal obtained from muscle activity is amplified, processed through a DAQ hardware system, and stimulation is triggered in an EMG-dependent manner. Electrical impulses are routed via a stimulator to the same access port, which delivers current to the cortical electrodes. Abbreviations: ECR = extensor carpi radialis; Bi = biceps; Trap = trapezius; TTL = transistor-transistor logic; DAQ = data acquisition; EMG = electromyogram.
Key Component: Chronic Implant
The chronic implant is made of two components which are soldered together peri-operatively: a Samtec® connector interface and the stimulation array. The Samtec® connector should be made beforehand, consisting of the connector itself, soldered to 2 cm 32 AWG gauge wires for soldering to the cortical array, and EMG wires soldered directly to the appropriate Samtec® pins. The stimulation array consists of four electrode leads made of platinum-iridium microwire, apposed together to a central cylindrical core, made of layers of heat shrink wrapped around a male electrical pin. For our experiments, the implant itself is made such that each electrode is equidistant from one another laterally by 1 mm. This ensures that the square area of the array itself encompasses a relatively large portion of the caudal forelimb area (CFA) of the motor cortex. The entire square array is encased in epoxy, to fix and insulate the electrodes. We recommend making the connector a few days prior to the implantation surgery, to leave time for quality control of all the connections and to perform two applications of epoxy to secure the wires. We soak both components in 70% alcohol the morning of the surgery for asepsis, but other sterilization methods are possible if stainless metal wire is used, including autoclaving.
Key Component: Connector Cable
The connecting cable from the rat implant to the commutator setup is, in our experience, often the weakest link in the entire hardware setup. This is unsurprising because the greatest torsion and other mechanical forces are placed on the wires within the cable. Rats will often try to bite the connecting cable once they are connected, to try to release themselves from the tether. Initially, we experienced poor EMG recording quality and permanent cable damage because one or more fine wires, including the plastic insulation within the connector cable were bitten off by the rat. We found that metal spring wire shielding provided sufficient protection of the cable from physical damage but also retained enough flexibility to permit the rat a high freedom of movement while tethered to the commutator.
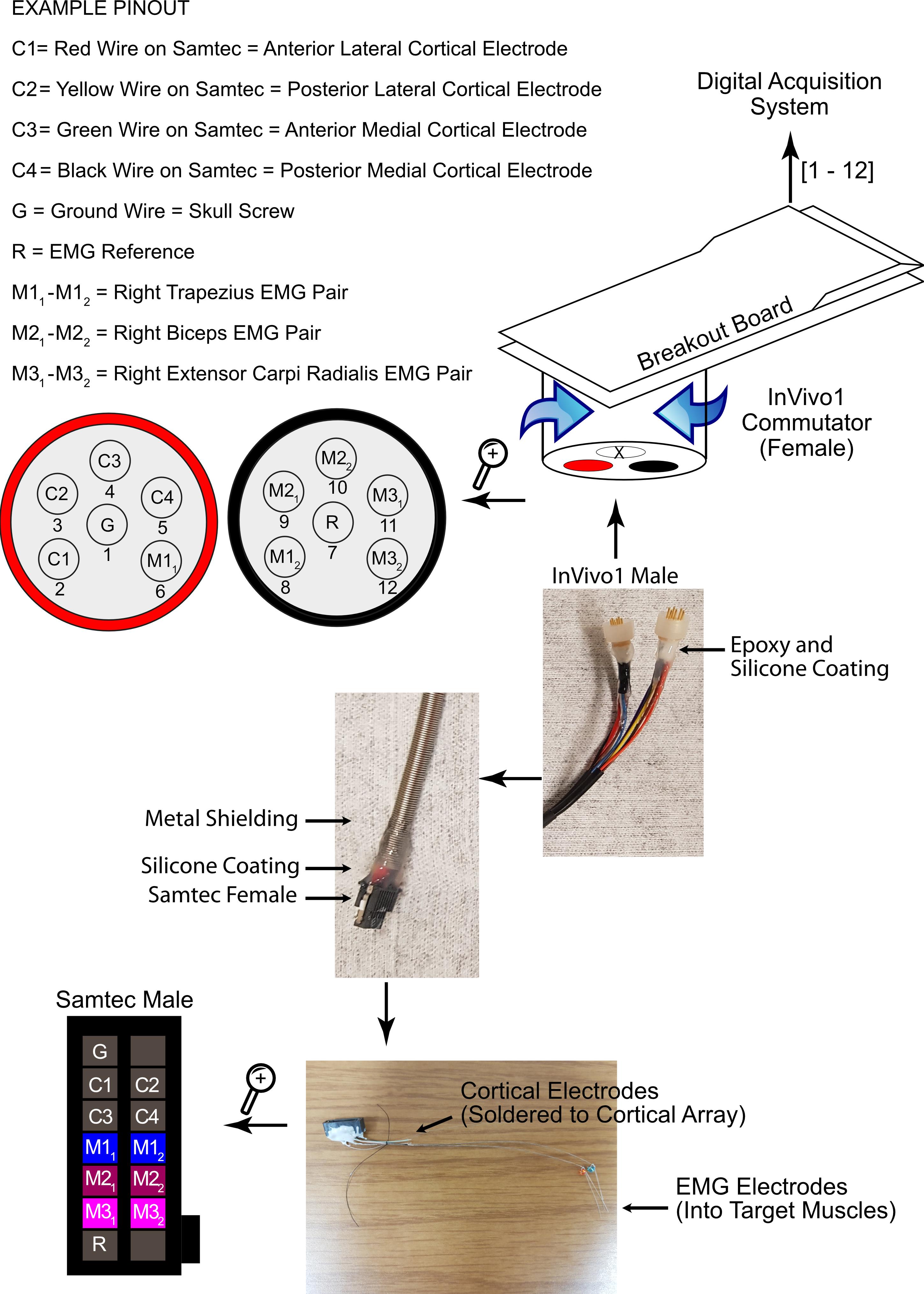
Figure 2. Commutator to Rat Hybrid Schematic. From top to bottom: A breakout board is used to connect the commutator to the DAQ system and the aforementioned computer system. The commutator is mounted on a rigid stand next to the rat’s cage and releases torsion on the connector cable, as the rat may rotate during locomotion and normal behavior. This maintains a secure electrical connection. Two out of the three sockets in the commutator are occupied according to the pinout provided. The metal shielding on the cable prevents physical damage to the wires within, and is a robust solution. The connection between the cable and the rat’s head is facilitated by one Samtec® female plugging into a Samtec® male connector port on the rat’s head, continuing the pinout described above. The Samtec® male connector’s designated muscle wires are implanted into the muscles during the first part of the surgery, and the designated cortex wires are soldered onto the corresponding wires on the cortical array during the latter part of the surgery.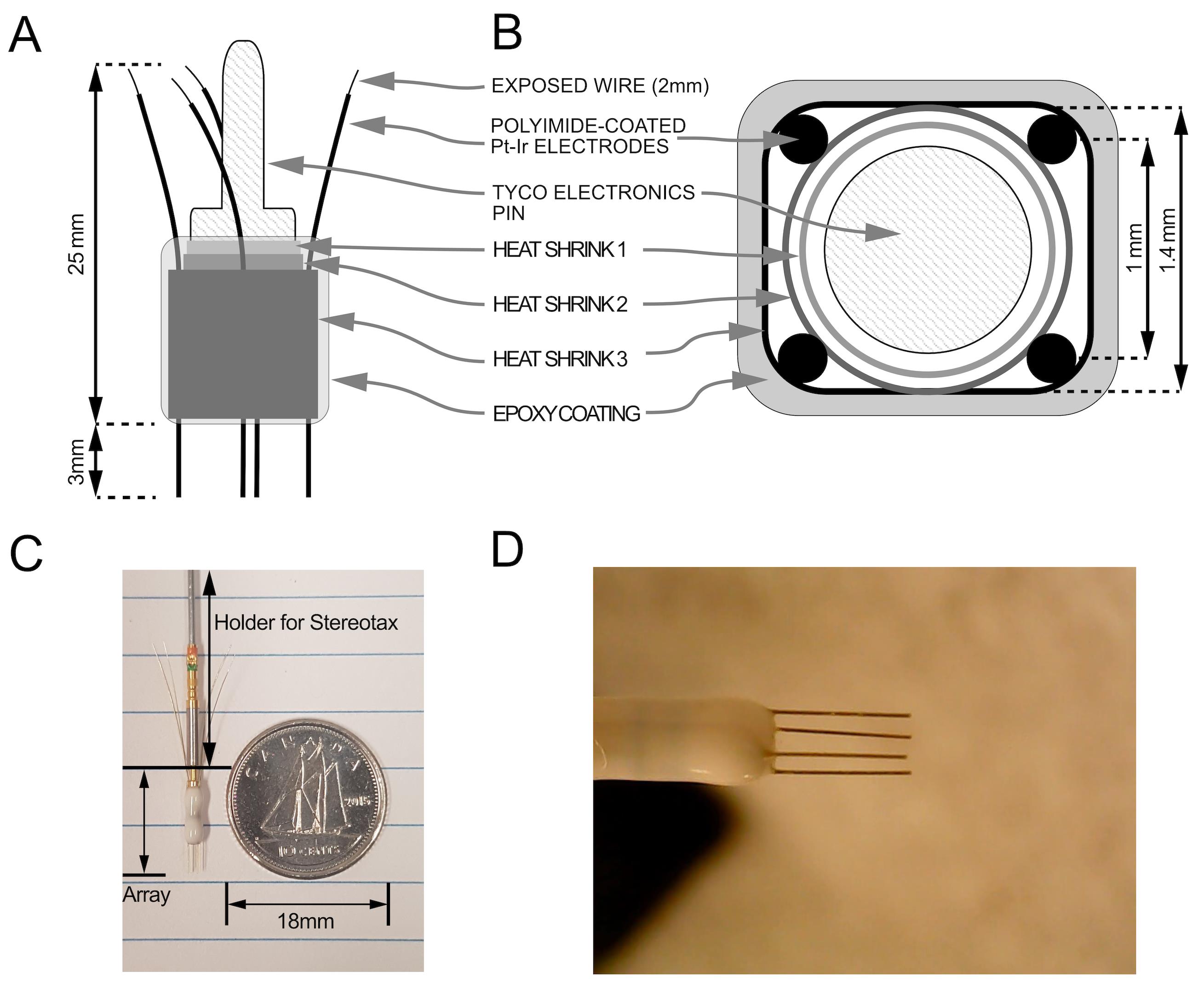
Figure 3. Intracortical electrode array blueprint (not to scale). A. Condensed array side view with measurements. B. Top view. C. Photograph of finished array and scale for reference. Array holder with female pin facilitates attachment to stereotaxic manipulator. D. Magnified view of finished array.
Equipment
To facilitate assembly, an electronic workbench with tools such as a soldering setup, low-magnification microscope, helping hands, crimpers, and flexible light source are required. Basic surgical tools, like scissors, blunt tweezers and scalpel (with flat Teflon® surface for removing insulation on EMG wires) are also necessary to manipulate the small components. A multimeter is helpful for verifying electrical contacts and the absence of shorts, as well as a heat gun.
Part A: Intracortical Stimulation Electrode Array (Peri-Operatively Soldered to Part B)
Chronic Implant80/20 Platinum-Iridium Wire (California Fine Wire Company, catalog number: 100168), 75 µm × 1 or equivalent
101 Epoxy adhesive (Omegabond, catalog number: OB-101-2) (with Part A and Part B mixable components)
“Iridium” Clear Polyolefin Heat Shrink Tubing, Min Expanded ID 0.066” (Cobalt Polymers, catalog number: X2-070-CLR), and 0.050” (Cobalt Polymers, catalog number: U2-055-CLR)
Tyco Electronics Series 90 Amplimite Connectors Pin (Tyco Electronics, catalog number: M39029/58-360)
Tyco Electronics Series 90 Amplimite Connectors Socket (Tyco Electronics, catalog number: M39029/57-354)
21 Gauge Stainless Steel Hypodermic Tubing, 0.032” OD (A-M Systems, catalog number: 843600)
Part B: Head Connector and EMG Electrodes (Port on Rat Head, and Electrodes in Muscles)
2 × 7 Male Connector (Samtec, catalog number: TFM-107-01-L-D-WT) × 1
Stainless Steel 7 Strand. 0.001” Bare, 0.0055” Coated, variable length × desired number of EMG electrodes (the number must be even to calculate a differential) and 5 cm reference electrode × 1 (A-M Systems, catalog number: 793200)
Generic Small Gauge (32 American Wire Gauge) Single Cored Electrical Wires, about 2 cm (maximum 4, or fewer desired cortical electrodes)
101 Epoxy adhesive (Omegabond, catalog number: OB-101-2) (with Part A and Part B mixable components).
Connector Cable (Rat to Commutator)
2 × 7 Female Connector and wire assembly with retention latches (Samtec, catalog number: SFSD-07-30C-G-12.00-SR) × 1
Silbione Silicone Medical Adhesive A-4300
Metal Spring Shielding (InVivo1, catalog number: 120 X .156)
12-Channel Component Cable with Open Ends (InVivo1, catalog number: 363/2-000)
Suitable Size Heat Shrink for Insulating Soldered Connections
101 Epoxy adhesive (Omegabond, catalog number: OB-101-2) (with Part A and Part B mixable components)
Stimulation and Recording Hardware (Commutator to Computer):
Laboratory Computer with MATLAB installation
A-M Systems 2,100 Stimulator × 2 or equivalent
Brownlee Precision Model 400 Amplifier or equivalent
Labchart Hardware System
Digital to Analog (DAC) SCB-68A Converter Digital Acquisition System
Invivo1 SL12C Double Brush Commutator
Associated Support Frames and BNC Connecting Cables
Software
PAS Analyzer Code at: https://github.com/ethierlab. Download repositories: PAS_interface_stim, PAS, Plotting, and Data_processing
MATLAB for Windows, Version 2018 or greater
LabChart Software Version 7 or higher
Latest drivers for stimulation and recording hardware, as well as DAQ System
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Ting, W. K. C., Burns, D., Huot-Lavoie, M. and Ethier, C. (2021). Assessment of Corticospinal Excitability in Awake Rodents Using EMG-Controlled Intracortical Stimulation. Bio-protocol 11(24): e4267. DOI: 10.21769/BioProtoc.4267.
- Ting, W. K., Huot-Lavoie, M. and Ethier, C. (2020). Paired Associative Stimulation Fails to Induce Plasticity in Freely Behaving Intact Rats. eNeuro 7(2): ENEURO.0396-19.2020.
分类
神经科学 > 感觉和运动系统
生物工程
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link











