- EN - English
- CN - 中文
A Native PAGE Assay for the Biochemical Characterization of G Protein Coupling to GPCRs
活性电泳分析测定G蛋白与GPCR偶联的生化表征
发布: 2021年12月20日第11卷第24期 DOI: 10.21769/BioProtoc.4266 浏览次数: 5252
评审: Kumiko OkazakiAnonymous reviewer(s)

相关实验方案
膜蛋白–配体相互作用研究的计算流程:以含 α5 亚基的 GABA(A) 受体为例
Syarifah Maisarah Sayed Mohamad [...] Ahmad Tarmizi Che Has
2025年11月20日 1789 阅读
Abstract
G protein-coupled receptors (GPCRs) are a large family of membrane-embedded receptors that have diverse roles in physiology and are major drug targets. GPCRs transduce an agonist binding signal across the membrane to activate intracellular heterotrimeric G proteins. The dynamic nature of the receptors and the complexity of their interactions with agonists and G proteins present significant challenges for biochemical studies. Most biochemical/biophysical methods that have been employed to study GPCR-G protein coupling require purified receptors and are technically difficult. Here, we provide a protocol for a relatively simple and time- and cost-effective membrane protein native PAGE assay, to visualize and biochemically characterize agonist-dependent coupling of detergent-solubilized GPCRs to purified G protein surrogate “mini-G” proteins, which stabilize the receptor in an active state. The assay was developed for our studies of the calcitonin receptor-like receptor, a class B GPCR that mediates the actions of calcitonin gene-related peptide and adrenomedullin peptide agonists. It does not require a purified receptor and it can be used in a screening format with transiently-transfected adherent mammalian cell cultures, to quickly identify detergent-stable complexes amenable to study, or in a quantitative format with membrane preparations, to determine apparent affinities of agonists for the mini-G-coupled receptor and apparent affinities of mini-G proteins for the agonist-occupied receptor. The latter provides a partial measure of agonist efficacy. The method should be applicable to other GPCRs, and has the potential to be adapted to the study of other challenging membrane proteins and their complexes with binding partners.
Graphic abstract:
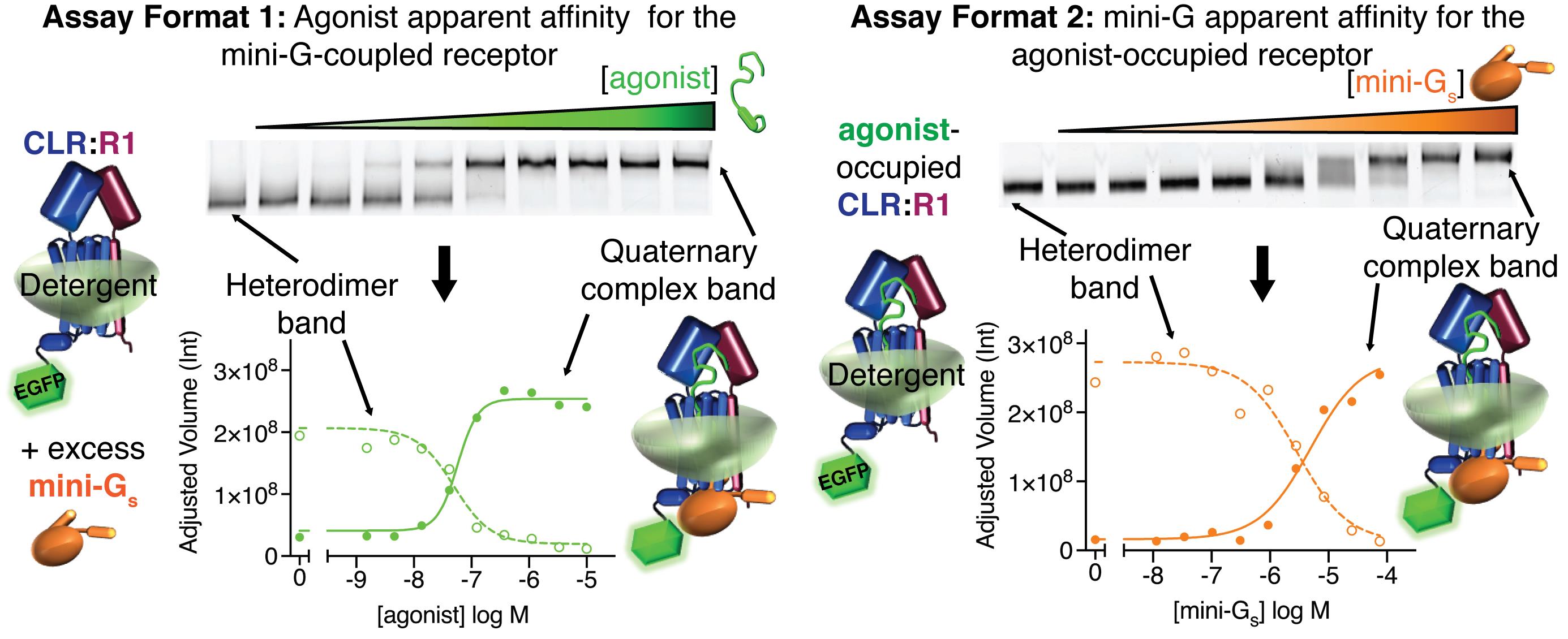
Visualizing agonist-dependent mini-G protein coupling and determining apparent binding affinities using the native PAGE assay quantitative formats.
Background
G protein-coupled receptors are a family of ~800 membrane-embedded receptors with critical physiologic roles in virtually every system of the human body and they are targeted by approximately one-third of FDA-approved therapeutics (Pierce et al., 2002; Sriram and Insel, 2018). Significant advances in biochemical, biophysical, and structural methods for the study of GPCRs have drastically improved our understanding of how an agonist-bound GPCR acts as a guanine nucleotide exchange factor, to activate intracellular heterotrimeric G proteins. Nonetheless, biochemical investigations of receptor-G protein coupling remain challenging due to the dynamic nature of the receptors and the complexity of their interactions with heterotrimeric G proteins, which are regulated by agonist binding to the GPCR, and guanine nucleotide binding to the G protein alpha subunit. Biochemical and biophysical methods that have been used to investigate this interaction include fluorescence- and nuclear magnetic resonance (NMR)-based methods (Yao et al., 2009; Gregorio et al., 2017; Huang et al., 2021). These methods are very powerful, but they are technically challenging and costly and require purified receptors in detergents or nanodiscs.
An alternative to working with G protein heterotrimers for biochemical studies is the use of “mini-G” proteins that were first developed as tools for GPCR structural studies (Carpenter and Tate, 2016; Nehmé et al., 2017). Mini-G proteins are minimal G protein alpha subunits that have enhanced stability in detergents and contain mutations that uncouple receptor binding from guanine nucleotide exchange, such that they trap GPCRs in an active state conformation, equivalent to that observed in structures of agonist-bound GPCRs in complex with nucleotide-free heterotrimer. Mini-G proteins are available for each of the four families of G protein alpha subunits. Biochemical assays for assessing mini-G interactions with GPCRs have been reported (Nehmé et al., 2017), including a fluorescent saturation binding assay using immobilized receptor, and an assay based on the fluorescence-detection size-exclusion chromatography (FSEC) technique (Kawate and Gouaux, 2006). These assays work with unpurified receptors in detergent-solubilized lysates and use GFP-tagged mini-G for detection.
We recently reported a novel biochemical assay for GPCR-mini-G coupling based on the high resolution clear native electrophoresis (hrCNE) technique, which is a membrane protein native-PAGE method compatible with fluorescently-labeled proteins (Wittig et al., 2007). We developed the assay during the course of our work to understand how calcitonin gene-related peptide and adrenomedullin peptide agonists and RAMP accessory proteins control G protein coupling of the class B GPCR calcitonin receptor-like receptor (CLR) (Roehrkasse et al., 2020). The method enables both rapid, cost-effective screening for detergent-stable GPCR-G protein complexes and quantitative studies of agonist-dependent receptor-mini-G coupling. It does not require a purified receptor. Instead, it uses an EGFP-tagged receptor transiently over-expressed in the mammalian HEK293S GnT1–; cell line. In the screening format, adherent cell cultures are directly solubilized with detergent, subjected to a simple centrifugation step, and the supernatants are analyzed by native-PAGE with visualization of the receptor by in-gel fluorescence imaging. Exogenous addition of agonist peptides and purified mini-G yields a mobility shift indicative of complex formation. In the quantitative assay format, crude membrane preparations are used to improve reproducibility and increase throughput. In the first quantitative format, the agonist is varied in the presence of a constant excess of mini-G to generate a binding curve that measures the apparent affinity of the agonist for the mini-G-coupled receptor. In the second quantitative format, mini-G is varied in the presence of a constant receptor-saturating concentration of agonist to generate a binding curve that measures the apparent affinity of mini-G for the agonist-occupied receptor. This second format provides a partial measure of agonist efficacy (Roehrkasse et al., 2020).
The native PAGE method proved to be a relatively simple, inexpensive, and highly versatile assay, which allowed us to investigate the biochemistry of agonist and G protein interactions with CLR:RAMP complexes. The screening and quantitative assay formats are described in detail here. We have also found the method to be very useful in a thermostability format, which is not described here, but can be found in our original report (Roehrkasse et al., 2020). This assay should be applicable to other GPCRs and may be valuable for the biochemical study of other challenging membrane proteins and their binding partners.
Materials and Reagents
Plasticware
15 ml conical tubes (VWR, catalog number: 76176-950)
48-well cell culture plate (Corning, Costar, catalog number: 3548)
Microfuge tubes (VWR, catalog number: 87003-294)
T-75 culture flask (Corning, catalog number: 43064)
Cell lines
HEK293S GnT1– (ATCC, catalog number: CRL-3022) (see Note 3)
Cell culture reagents
DMEM with 4.5 g/L Glucose and L-Glutamine (Lonza, catalog number: 12-604Q)
Fetal Bovine Serum (FBS) (Gibco, catalog number: 16000-044)
Non-Essential Amino Acids (NEAA) (Gibco, catalog number: 11140-050)
Penicillin/Streptomcyin (Gibco, catalog number: 15140-122)
Phosphate Buffered Saline (PBS) (Gibco, catalog number: 10010-023)
Polyethylenimine, branched (PEI) (Sigma-Aldrich, catalog number: 408727-100 ml)
Salts – stored at room temperature
Ammonium persulfate (APS) (Bio-Rad, catalog number: 1610700)
Calcium Chloride (CaCl2) (Sigma-Aldrich, catalog number: 223506-500G)
HEPES Sodium Salt (Sigma-Aldrich, catalog number: H7006-500G)
Potassium Chloride (KCl) (Sigma-Aldrich, catalog number: P9333-500G)
Magnesium Chloride (MgCl2) (Sigma-Aldrich, catalog number: 442611-500G)
Sodium Chloride (NaCl) (EMD Millipore, catalog number: SX0420-5)
Valproic acid sodium salt (Sigma-Aldrich, catalog number: P4543-25G)
Alcohols
Isoproponol (VWR, BDH, catalog number: BDH1133-4LG)
Methanol (Supelco, EMD Millipore, catalog number: MX0475-1)
Solubilizing chemicals – stored at -20°C
Lauryl Maltose Neopentyl Glycol (LMNG) (Anatrace, catalog number: NG310-25GM)
Cholesteryl Hemisuccinate (CHS) (Anatrace, catalog number: CH210-5GM)
Other Chemicals
6-amino hexanoic acid (Sigma-Aldrich, catalog number: 07260-1KG)
30% Acrylamide/Bis-acrylamide 29:1 (Acryl/Bis) (Bio-Rad, catalog number: 1610156)
Fatty-Acid-Free BSA (FAF BSA) (PAA Laboratories, catalog number: K35-002)
Glutathione oxidized (GSSG) (Sigma-Aldrich, catalog number: G4376-10G)
Glutathione reduced (GSH) (Sigma-Aldrich, catalog number: G4251-25G)
Imidazole (EMD Millipore, catalog number: 5720-500GM)
Sodium Hydroxide (NaOH) (VWR, BDH, catalog number: BDH3247-1)
Tetramethylenediamine (TEMED) (Bio-Rad, catalog number: 161-0800)
Tricine (Sigma-Aldrich, catalog number: T0377-250G)
Protease Inhibitor tablets (Thermo Scientific, Pierce, catalog number: A32955)
Peptides
Human α calcitonin gene-related peptide (Bachem, catalog number: 4013281)
Human adrenomedullin (Bachem, catalog number: 4034489)
Human adrenomedullin2/intermedin (Bachem, catalog number: 4044529)
Enzymes
Apyrase (New England BioLabs, catalog number: M0398L)
Membrane preparations and purified proteins
Crude membrane preparation from HEK293S GnT1– cells transiently co-expressing tagged MBP-CLR-EGFP and MBP-RAMP, using the pHLsec expression vector (Aricescu et al., 2006); the preparation method was published in Roehrkasse et al. (2020) – flash-frozen in liquid nitrogen and stored at -80°C. The final preparationss are stored in 25 mM HEPES, pH 7.5, 10% (v/v) glycerol, 25 mM NaCl, 2 mM MgCl2, and 1× protease inhibitor tablet. Protein concentrations are ~4-6 mg/ml with the receptor at ~100 nM.
Purified H6-SUMO-mini-G proteins (mini-Gs, mini-Gs/i, and mini-Gs/q were used; the present protocol utilizes example data from miniGs); methods published in Carpenter and Tate (2016 and 2017), Nehmé et al. (2017) , and Roehrkasse et al. (2020) – stored at -80°C. The purified proteins are stored at ~25-30 mg/ml in a buffer of 25 mM HEPES, pH 7.5, 50% (v/v) glycerol, 150 mM NaCl, 0.5 mM DTT, 1 mM MgCl2, and 1 μM GDP (see Notes 1 and 2).
High resolution clear native PAGE gel (see Recipes)
4× Binding Buffer (see Recipes)
4× Detergent Buffer (see Recipes)
Running Buffers (see Recipes)
Culture media (see Recipes)
Transfection media (see Recipes)
100 mM GSH and GSSG redox buffer stocks (see Recipes)
Equipment
Mini-PROTEAN Electrophoresis apparatus (Bio-Rad, model: 1658001FC)
Chemidoc MP imager (Bio-Rad, model: 12003154)
CO2 incubator (NuAire, model: NU-5800)
Imager tray (Bio-Rad, catalog number: 12003028)
Rocking shaker (Reliable Scientific, model: 55)
Tube tumbler (Labnet International, model: H5500)
Benchtop Micro centrifuge (Eppendorf, model: 5415R)
Software
ImageLab (Bio-Rad, https://tinyurl.com/2v4t44re)
Prism (GraphPad, https://www.graphpad.com/)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Roehrkasse, A. M., Karim, J. A. and Pioszak, A. A. (2021). A Native PAGE Assay for the Biochemical Characterization of G Protein Coupling to GPCRs. Bio-protocol 11(24): e4266. DOI: 10.21769/BioProtoc.4266.
- Roehrkasse, A. M., Warner, M. L. Booe, J. M., and Pioszak, A. A. (2020). Biochemical characterization of G protein coupling to calcitonin gene-related peptide and adrenomedullin receptors using a native PAGE assay. J Biol Chem 295(28): 9736-9751.
分类
生物化学 > 蛋白质 > 相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
提问指南
+ 问题描述
写下详细的问题描述,包括所有有助于他人回答您问题的信息(例如实验过程、条件和相关图像等)。
Share
Bluesky
X
Copy link