- EN - English
- CN - 中文
A Simple Method for the Isolation and in vitro Expansion of Highly Pure Mouse and Human Satellite Cells
一种分离和体外扩增高纯度小鼠和人类卫星细胞的简单方法
发布: 2021年12月05日第11卷第23期 DOI: 10.21769/BioProtoc.4238 浏览次数: 3971
评审: Alessandro DidonnaSimab KanwalSaskia F. Erttmann
Abstract
Satellite cells (SCs) are muscle stem cells capable of regenerating injured muscle. The study of their functional potential depends on the availability of methods for the isolation and expansion of pure SCs, which retain myogenic properties after serial passages in vitro. Here, we describe a protocol for the isolation and in vitro expansion of highly pure mouse and human SCs based on ice-cold treatment (ICT). The ICT is carried out by briefly incubating the dish containing a heterogeneous mix of adherent muscle mononuclear cells on ice for 15-30 min, which leads to the detachment only of the SCs, and gives rise to SC cultures with 95-100% purity. This approach can also be used to passage the cells, allowing SC expansion over extended periods of time without compromising their proliferation or differentiation potential. Overall, the ICT method is cost-effective, accessible, technically simple, reproducible, and highly efficient.
Graphic abstract:
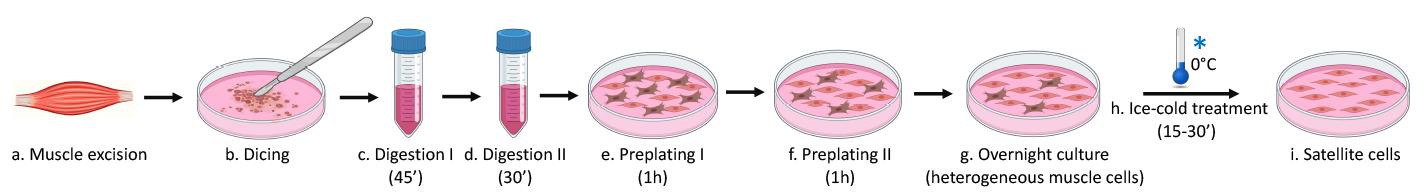
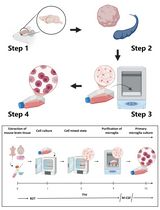
Figure 1. Satellite cell isolation using the ice-cold treatment method.
Background
The exceptional regenerative ability of skeletal muscle is primarily due to a resident population of stem cells called satellite cells (SCs) (Mauro, 1961; Chang and Rudnicki, 2014; Wang et al., 2014). The study of their functional potential depends on the availability of methods for the isolation and expansion of highly pure SCs with preserved myogenic properties after serial passages in vitro (Danoviz and Yablonka-Reuveni, 2012; Keire et al., 2013; Syverud et al., 2014).
Presently, there are three main methods commonly used for the isolation of SCs: pre-plating, fluorescence activated cell sorting (FACS), and magnetic bead isolation.
The pre-plating method is based on the differing adhesive properties of muscle cells, with SCs being the least adherent (Gharaibeh et al., 2008; Danoviz and Yablonka-Reuveni, 2012; Keire et al., 2013; Syverud et al., 2014). Although cheap and straightforward to perform, this method’s main disadvantages are that it is time consuming and gives rise to cultures of variable purity, often with fibroblast contamination and overgrowth by day 7 of culture (Keire et al., 2013).
The FACS sorting method sorts muscle mononuclear cells pre-labeled with SC specific antibodies (Fukada et al., 2004; Sherwood et al., 2004; Montarras et al., 2005; Pasut et al., 2012; Chapman et al., 2013; Liu et al., 2015). At present, FACS sorting is the gold standard for the isolation and study of SCs. Nevertheless, there are several disadvantages to this method, including high cost and the requirement for a FACS sorter instrument. Moreover, this method is time consuming, requires expertise to perform, and cell purity can be variable. The cell labeling step that precedes the sorting procedure can potentially stress or damage the cells, decrease their viability, or alter their functional properties in vitro (Syverud et al., 2014).
Finally, the third method is based on magnetic cell separation (MACS) and uses magnetic columns and SC specific magnetic bead kits (Blanco-Bose et al., 2001). Because this method assumes that all the other cell types are successfully removed from the muscle cell preparation, it is less precise than the FACS sorting method. This method is expensive to perform, time consuming, and stressful for the cells. As for the other two methods, cell purity is variable, and often the SC cultures become overgrown by fibroblasts by day 7 (Keire et al., 2013; Syverud et al., 2014).
The ideal SC isolation technique would permit isolation of pure SCs with minimal manipulation, producing cells that could be expanded ex vivo without losing their stemness and regenerative capacity. Here, we describe a protocol for ice-cold treatment (ICT); this is a simple, inexpensive, and efficient method for the isolation and long-term expansion of highly pure mouse and human SCs that preserves their myogenic potential (Benedetti et al., 2020). In terms of purity of the isolated cell population, the ICT method outperforms others, such as pre-plating or magnetic bead isolation. Furthermore, it is fast and easy to perform—apart from the time required for enzymatic digestion (1.5 h), it involves minimal manipulation of the cells. Another major advantage of the ICT method is that it doubles up as a very gentle passaging technique, allowing long-term serial expansion of SCs ex vivo without altering their proliferation and differentiation properties. In turn, this drastically reduces the number of mice or muscle biopsies required to obtain a sufficient number of cells (Benedetti et al., 2021). The ICT method permits growing mouse and human SCs to be passaged at least 10 times, expanding their number 150- and 300-fold, respectively (Benedetti et al., 2021). This represents a clear advantage over the most commonly used passaging reagent (trypsin), which typically accelerates the differentiation of passaged SCs after only two passages (Danoviz and Yablonka-Reuveni, 2012; Benedetti et al., 2020 and 2021; Fiore et al., 2020).
Overall, the cost-effectiveness, accessibility, and technical simplicity of this protocol, as well as its remarkable efficiency, represent major improvements over existing protocols. The next step will be to test this protocol for the isolation and expansion of stem cells from tissues other than muscle.
Materials and Reagents
Scalpel and surgical blades (Securelab, size 24)
70 µm cell strainers (Falcon, catalog number, catalog number: 352350)
40 µm cell strainers (Falcon, catalog number, catalog number: 352340)
100 mm tissue culture dishes (Falcon, catalog number: 430167)
35 mm tissue culture dishes (Falcon, catalog number: 353001)
60 mm tissue culture dishes (Falcon, catalog number: 353002)
50 ml polypropylene centrifuge tubes (Falcon, catalog number: 352098)
10 ml serological pipettes (Falcon, catalog number: 357551)
Dissection boards (Styrofoam board)
Cover slips 24 × 50 (Menzel, catalog number: 15737592)
Pipette tips (Corning)
Parafilm (Sigma, catalog number: P7793)
WT C57BL/6J mice aged 4-6 weeks (The Jackson Laboratory)
Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich, catalog number: D5671)
Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A7030)
Hydrophobic PAP pen for immunostaining (Sigma-Aldrich, catalog number: Z377821)
Human muscle biopsies [i.e., gluteus maximus biopsies obtained from patients undergoing surgery; Benedetti et al. (2021)]
Vectashield mounting medium (Vector Laboratories, catalog number: H-1000-10)
Hoechst 33342 staining dye (Abcam, catalog number: ab228551)
Primary antibodies:
Pax7 (Developmental Studies Hybridoma Bank)
MyoD (Santa Cruz Biotechnology, catalog number: sc-760)
MyoG (F5D) (Developmental Studies Hybridoma Bank)
MyHC (MF20) (Developmental Studies Hybridoma Bank)
Fluorescently labeled secondary antibodies:
Goat anti-rabbit Alexa Fluor 488 (1:1,000, Abcam, catalog number: 150077)
Goat anti-mouse Alexa Fluor 555 (1:1,000, Thermo Fisher Scientific, catalog number: A28180)
0.1% gelatin (Stem Cell Technologies, catalog number: 07903)
L-glutamine (Sigma-Aldrich, catalog number: 59202C)
Chicken embryo extract (Seralab, catalog number: CE-650-J)
Goat serum (Sigma-Aldrich, catalog number: G9023)
Horse serum (Thermo Fisher Scientific, catalog number: 26050088)
Fetal bovine serum (FBS) (Sigma-Aldrich, catalog number: F2442), heat inactivated at 56°C for 30 min
Dulbecco’s Phosphate-Buffered Saline (PBS) with MgCl2 and CaCl2 (Sigma-Aldrich, catalog number: D8662)
Dulbecco’s Phosphate-Buffered Saline (PBS), modified without MgCl2 and CaCl2 (Sigma-Aldrich, catalog number: D8537)
Penicillin-streptomycin (pen/strep) solution (Sigma-Aldrich, catalog number: P0781)
Gentamycin solution (Sigma-Aldrich, catalog number: G1397)
Formaldehyde solution 4% (Sigma-Aldrich, catalog number: 1004968350)
Methanol (Sigma-Aldrich, catalog number: 34860)
Collagenase type II (Sigma-Aldrich, catalog number: C6885)
Collagenase/dispase (Roche, catalog number: 11097113001)
Ethanol (Sigma-Aldrich, catalog number: 51976)
70% ethanol (see Recipes)
Collagenase type II digestion solution (see Recipes)
Collagenase/dispase digestion solution (see Recipes)
Growth medium (see Recipes)
Differentiation medium (see Recipes)
Neutralization buffer (see Recipes)
Hoechst solution (see Recipes)
Equipment
Microsurgery scissors (Fine Science Tool, catalog number: 14184-09)
Microsurgery tweezers (Fine Science Tool, catalog number: 11252-00)
Pipettes (Gilson, P10, 20, 200, 1000)
Humidified chamber (prepared by wetting paper towels with distilled water and placing them in a plastic container with a lid)
Centrifuge (Eppendorf, model: 5702)
Vertical Autoclave (Falc, model: ATV80)
Temperature regulated shaking water bath (GLS, catalog number: 1083)
Biosafety cabinet (Gelaire, model: BSB4 A)
Laboratory chemical fume hood (ESCO, Frontier Acela)
CO2 incubator Thermo Forma (Thermo Fisher Scientific, model: 3110)
Zeiss Axioskop 2 Plus microscope (Carl Zeiss)
Phase-contrast microscope (Nikon Eclipse, model: TS100)
Pipet Controllers for serological pipettes (Falcon)
Hemocytometer counting chamber Neubauer improved (BLAUBRAND, model: BR717810)
Ice machine
Software
ZEISS ZEN 2 Blue edition (Carl Zeiss) (downloa from https://www.zeiss.com/microscopy/int/products/microscope-software.html)
ImageJ 1.53a (download from https://imagej.nih.gov/ij/download.html)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Benedetti, A., Cera, G., De Meo, D., Villani, C., Bouche, M. and Lozanoska-Ochser, B. (2021). A Simple Method for the Isolation and in vitro Expansion of Highly Pure Mouse and Human Satellite Cells. Bio-protocol 11(23): e4238. DOI: 10.21769/BioProtoc.4238.
分类
干细胞 > 成体干细胞 > 肌肉干细胞
细胞生物学 > 细胞分离和培养 > 细胞分离
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link