- EN - English
- CN - 中文
Fixation and Immunostaining of Endogenous Proteins or Post-translational Modifications in Caenorhabditis elegans
秀丽隐杆线虫内源性蛋白质的固定和免疫染色或翻译后修饰
(*contributed equally to this work) 发布: 2021年10月05日第11卷第19期 DOI: 10.21769/BioProtoc.4172 浏览次数: 3828
评审: DURAI SELLEGOUNDERManish ChamoliAnonymous reviewer(s)

相关实验方案

用Argonaute NRDE-3标记活性转录位点—图像秀丽隐杆线虫体内的活性转录位点
Antoine Barrière and Vincent Bertrand
2022年06月05日 2685 阅读
Abstract
Although the advent of genetically-encoded fluorescent markers, such as the green fluorescent protein (GFP; Chalfie et al., 1994), has enabled convenient visualization of gene expression in vivo, this method is generally not effective for detecting post-translational modifications because they are not translated from DNA sequences. Genetically-encoded, fluorescently-tagged transgene products can also be misleading for observing expression patterns because transgenes may lack endogenous regulatory DNA elements needed for precise regulation of expression that could result in over or under expression. Fluorescently-tagged proteins created by CRISPR genome editing are less prone to defective expression patterns because the loci retain endogenous DNA elements that regulate their transcription (Nance and Frøkjær-Jensen, 2019). However, even CRISPR alleles encoding heritable fluorescently-tagged protein markers can result in defects in function or localization of the gene product if the fluorescent tag obstructs or otherwise interferes with important protein interaction domains or affects the protein structure.
Indirect immunofluorescence is a method for detecting endogenous gene expression or post-translational modifications without the need for transgenesis or genome editing. Here, we present a reliable protocol in which C. elegans nematodes are fixed, preserved, and permeabilized for staining with a primary antibody to bind proteins or post-translational modifications, which are then labeled with a secondary antibody conjugated to a fluorescent dye. Use of this method may be limited by the availability of (or ability to generate) a primary antibody that binds the epitope of interest in fixed animals. Thousands of animals are simultaneously subjected to a series of chemical treatments and washes in a single centrifuge tube, allowing large numbers of identically-treated stained animals to be examined. We have successfully used this protocol (O’Hagan et al., 2011 and 2017; Power et al., 2020) to preserve and detect post-translational modifications of tubulin in C. elegans ciliated sensory neurons and to detect non-modified endogenous protein (Topalidou and Chalfie, 2011).
Keywords: Fixation (固定)Background
The ability to detect the presence and localization of endogenous biological molecules or epitopes in organisms is essential to understand their function. Previous methods of fixation and permeabilization of C. elegans for immunofluorescent detection of molecules include both freeze cracking and tube fixations (Finney and Ruvkun, 1990; Miller and Shakes, 1995; Duerr et al., 2006). Here, we present a robust method (see overview in Figure 1), modified from the one described by Finney and Ruvkun (1990).
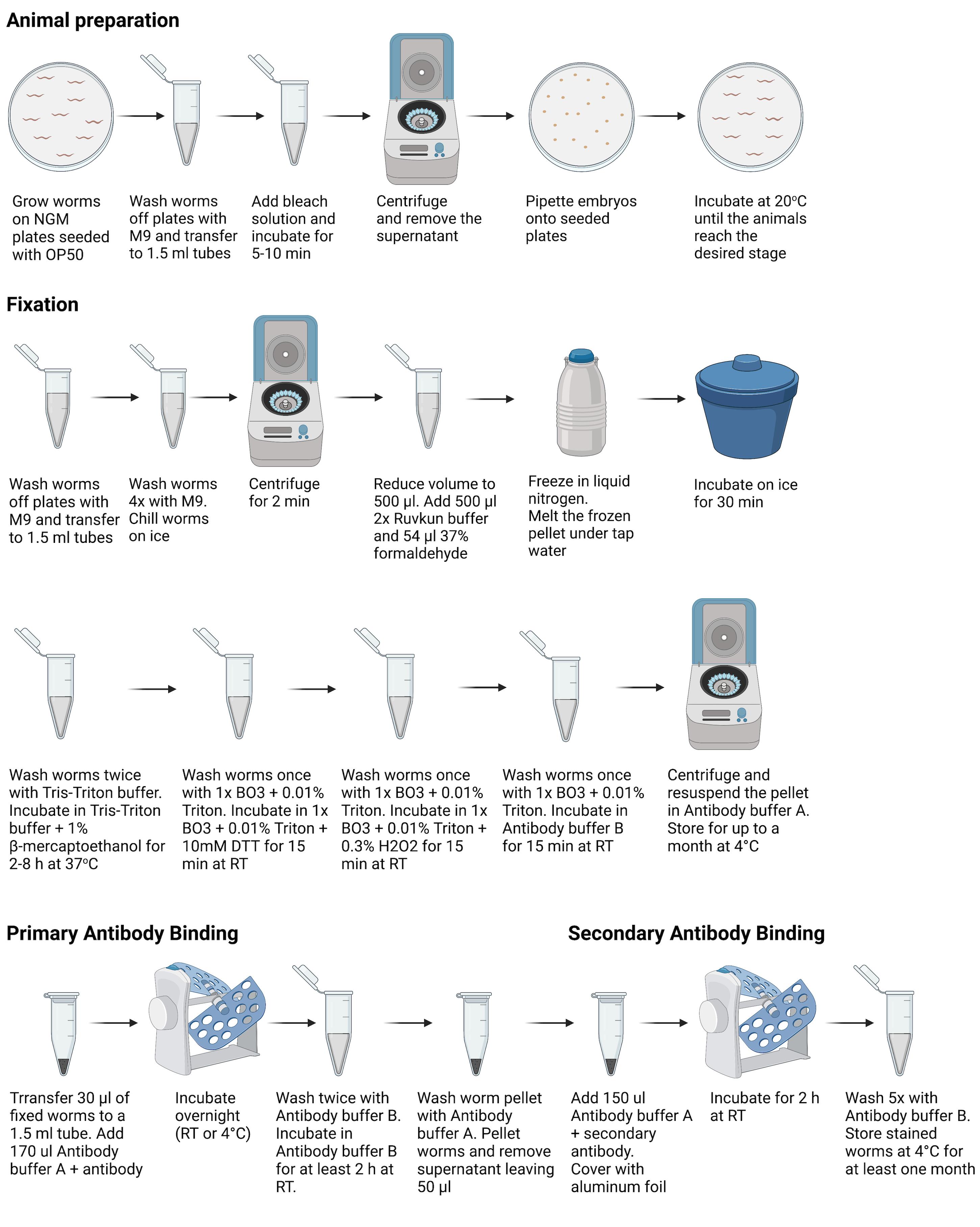
Figure 1. Overview of procedures used for animal preparation, fixation, and primary and secondary antibody binding. Figure 1 was created with Biorender.com.
Materials and Reagents
C. elegans strains of interest (https://cgc.umn.edu/)
OP50 E. coli strain (https://cgc.umn.edu/)
NGM plates seeded with OP50
PBS/phosphate Buffered Saline, 1× Powder, pH 7.4 (Fisher Scientific, catalog number: BP661-10)
Tris Base (Sigma-Aldrich, catalog number: 648311)
EDTA (Sigma-Aldrich, catalog number: E9884)
EGTA (Sigma-Aldrich, Calbiochem, catalog number: 324626)
Spermidine trihydrochloride (Sigma-Aldrich, catalog number: S2501)
PIPES (Sigma-Aldrich, catalog number: P1851)
BME/β-mercaptoethanol (Sigma-Aldrich, catalog number: M6250)
DTT/dithiothreitol (Fisher Scientific, catalog number: FERR0861). Store at 4°C, or in aliquots at -20°C
H2O2/Hydrogen Peroxide 30% with stabilizer (Sigma-Aldrich, catalog number: H1009)
H3BO3/Boric Acid Powder 99.5% (Fisher Scientific, catalog number: 18-609-176)
Formaldehyde Solution, 37% (Sigma-Aldrich, catalog number: 252549). Store at 4°C
Triton X-100 non-ionic detergent (Sigma-Aldrich, catalog number: X100)
BSA (Sigma-Aldrich, catalog number: A2153). Store at 4°C, or in aliquots at -20°C
NaN3/Sodium Azide (Sigma-Aldrich, catalog number: S2002)
GT335 (AdipoGen Life Sciences, catalog number: AG-20B-0020-C100). Store at -20°C
Alexa-fluor 568-conjugated donkey anti-mouse secondary antibody (Fisher Scientific, Invitrogen, catalog number: A10037). Store in the dark at 4°C
Sodium hypochlorite 5.65-6% solution (Fisher Scientific, catalog number: SS290-4) or Clorox bleach (~7.5% sodium hypochlorite)
Potassium hydroxide (KOH) (Fisher Scientific, catalog number: P251-500)
Agarose (Fisher Scientific, catalog number: 16-520-050)
2× Ruvkun Buffer (see Recipes)
Tris-Triton buffer (see Recipes)
20× BO3 buffer (see Recipes)
1× BO3 + 0.01% Triton buffer (see Recipes)
Antibody buffer A (see Recipes)
Antibody buffer B (see Recipes)
M9 solution (see Recipes)
5 M KOH (see Recipes)
Tris-Triton buffer +1% β-mercaptoethanol (see Recipes)
1 M DTT solution (see Recipes)
1× BO3 + 0.01% Triton buffer + 10 mM DTT (see Recipes)
1× BO3 + 0.01% Triton buffer + 0.3% H2O2 (see Recipes)
Liquid 2% agarose (see Recipes)
Equipment
Note: Equipment similar to the items described below is required.
Platform Rocker (Fisher Scientific, Thermo Scientific Vari-Mix, catalog number: 09-047-113Q)
Microcentrifuge (Fisher Scientific, Thermo Scientific Sorvall Legend 21, catalog number: 75-77-2488)
Compound epifluorescence or confocal microscope with 63× (NA 1.4) or 100× (NA 1.4) oil-immersion objectives
Digital microscope camera (Examples of microscope cameras we have used successfully for this protocol include:
Retiga-SRV Fast 1394 digital camera
Photometrics Cascade 512B CCD camera
Hamamatsu C11440-42U ORCA-Flash4.0 LT Digital CMOS camera
Photometrics CoolSNAP HQ2-FW camera
Microscope slides (Fisher Scientific, catalog number: 22-265446)
Carl Zeiss High Performance Coverslips (Fisher Scientific, catalog number: 10474379)
Glass Pasteur pipettes (Fisher Scientific, catalog number: 13-678-20A)
Eyelash glued to a toothpick
Software
ImageJ (NIH, https://imagej.nih.gov/ij/)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
O'Hagan, R. and Topalidou, I. (2021). Fixation and Immunostaining of Endogenous Proteins or Post-translational Modifications in Caenorhabditis elegans. Bio-protocol 11(19): e4172. DOI: 10.21769/BioProtoc.4172.
分类
细胞生物学 > 细胞染色 > 蛋白质
免疫学 > 免疫细胞染色
细胞生物学 > 细胞成像 > 荧光
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link











