- EN - English
- CN - 中文
Isolation of Nasal Brush Cells for Single-cell Preparations
用于单细胞制备的鼻刷细胞的分离
发布: 2021年09月20日第11卷第18期 DOI: 10.21769/BioProtoc.4163 浏览次数: 5666
评审: Laura CampisiSonal Patel PatelMarie BoutetAnonymous reviewer(s)
Abstract
Solitary chemosensory epithelial cells are scattered in most mucosal surfaces. They are referred to as tuft cells in the intestinal mucosa, brush cells in the trachea, and solitary chemosensory and microvillous cells in the nasal mucosa. They are the primary source of IL-25 in the epithelium and are also engaged in acetylcholine generation. We recently demonstrated that nasal solitary chemosensory (brush) cells can generate robust levels of cysteinyl leukotrienes in response to stimulation with calcium ionophore, aeroallergens, and danger-associated molecules, such as ATP and UTP, and this mechanism depends on brush cell expression of the purinergic receptor P2Y2. This protocol describes an effective method of nasal brush cell isolation in the mouse. The method is based on physical separation of the mucosal layer of the nasal cavity and pre-incubation with dispase, followed by digestion with papain solution. The single cell suspension obtained this way contains a high yield of brush cells for fluorescence-activated cell sorting (FACS), RNA-sequencing, and ex vivo assays.
Graphic abstract:
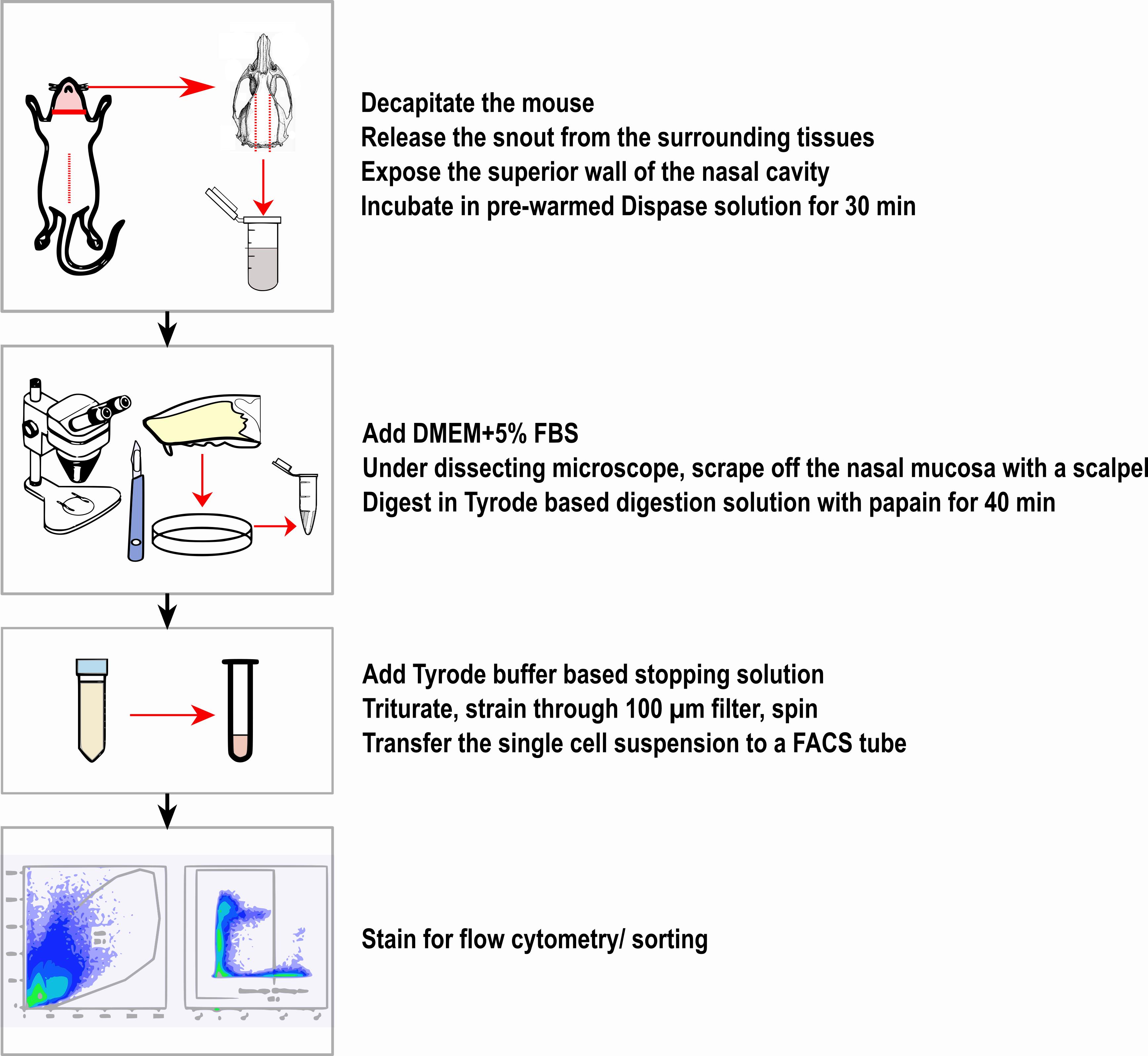
Workflow of nasal digestion for brush cell isolation.
Background
Brush cells are chemosensory epithelial cells best known for their expression of bitter taste receptors (Finger et al., 2003; Krasteva and Kummer, 2012). Brush cells are also be referred to as tuft cells and have been found in the airways (Krasteva and Kummer, 2012), gastrointestinal tract (Howitt et al., 2016), and urinary epithelium (Deckmann et al., 2014). Morphologically, brush cells are characterized by apical microvilli that form a tuft-like projection on the cell surface, extending to the mucosal lumen (Rhodin and Dalhamn, 1956; Reid et al., 2005). Protective functions of brush cells have been most closely linked to their generation of acetylcholine leading to activation of peptidergic sensory nerve fibers (Krasteva et al., 2011). We have previously reported a role for brush cell-derived IL-25 in eliciting type 2 inflammation and epithelial cell remodeling in the lung (Bankova et al., 2018). We recently reported robust generation of pro-inflammatory lipid mediators termed cysteinyl leukotrienes by nasal brush cells in response to aeroallergens and ATP through the purinergic receptor P2Y2 (Ualiyeva et al., 2020), suggesting potent functions for these cells in directing immune responses in the airways.
In the nose, the mucosal lining is composed of olfactory and respiratory epithelia (Adams, 1972). In mice, the olfactory epithelium lines up to 50% of the total nasal mucosa, overlying the middle and superior turbinates and the caudal/posterior area of the nasal septum, while the respiratory epithelium covers around 45% of the nasal cavity in more rostral/anterior parts (Gross et al., 1982; Chamanza and Wright, 2015). Solitary chemosensory cells in the respiratory mucosa of the nose respond to irritants and bacteria and are established as the nasal equivalent of tracheal brush cells in mice (Finger et al., 2003; Sbarbati and Osculati, 2003) and humans (Lee et al., 2014; Kohanski et al., 2018). Microvillous cells are related cells found in the apical olfactory epithelium, above the layer of olfactory sensory neurons, and supporting sustentacular cells (Hansen and Finger, 2008). Both microvillous cells and solitary chemosensory cells express Chat, choline acetyltransferase, an enzyme necessary for the synthesis of the neurotransmitter acetylcholine (Krasteva et al., 2011), Trpm5, a transient receptor potential gene ubiquitous in chemosensory cells (Pérez et al., 2002; Kaske et al., 2007; Zhang et al., 2007; Ogura et al., 2011; Genovese and Tizzano, 2018), and depend on Pou2f3, a transcription factor required for differentiation of all chemosensory cells in the nose, trachea, and intestine (Ohmoto et al., 2013; Yamaguchi et al., 2014; von Moltke et al., 2016).
Our recent study using fluorescence-activated cell sorting (FACS) based on the fluorescent reporter expression of Chat (ChAT-eGFP) and size and granularity characteristics [forward scatter (FSC) and side scatter (SSC)] allowed us to distinguish the olfactory-enriched microvillar cells from the respiratory-enriched solitary chemosensory cells. We confirmed by bulk RNA-sequencing (RNA-seq) that both populations belong to the larger family of chemosensory tuft/brush cells and are mostly distinguished by their varied expression of taste receptors. This protocol describes our method for isolation of a mixed population of nasal chemosensory brush cells providing high yields of ~20,000/mouse, which can be further characterized by FACS and RNA-seq for investigation of the similarities and differences between these cell types in the nasal cavity.
Several groups have reported successful isolation of solitary chemosensory cells from the respiratory nasal mucosa and microvillar cells from the olfactory mucosa. Gulbransen et al. (2008) used a Tyrode-based solution supplemented with 20 U/ml of papain to isolate solitary chemosensory cells from the respiratory mucosa of TRPM5-GFP fluorescent reporter mice. Several hundred viable cells were used for calcium flux studies in response to bitter tasting agonists. Lin et al. (2008) isolated solitary chemosensory cells from the anterior respiratory epithelium with a Ringer-based solution with 10-30 U/ml of papain to measure changes in intracellular calcium levels in single cells in response to odorous irritants. Ogura et al. (2011) isolated microvillar cells from the main olfactory epithelium of ChAT-eGFP and TRPM5-GFP mice with 4 U/ml of papain for 3-5 min at room temperature and showed increased calcium flux from freshly isolated cells in response to ATP, soil bacterium lysate, and denatonium benzoate. Although no information was provided for the total yields of recovered solitary chemosensory or microvillous cells with the described protocols, each used <100 cells for live imaging for calcium flux. We tested a comparable concentration of papain (26 U/ml) to isolate brush cells from the trachea and found that pre-incubation of the trachea with high dose dispase (16 U/ml) results in a 20-fold increase of isolated brush cell yields compared to digestion with papain alone (Ualiyeva et al., 2019).
Here, we describe a step-by-step protocol for isolation of brush cells from the murine snout of ChAT-eGFP fluorescent transgenic mice, with enhanced green fluorescent protein expression dictated by Chat. Choline acetyltransferase is highly expressed in both subsets of nasal chemosensory brush cells, in tracheal, intestinal, and urethral brush/tuft cells and specifically enriched in these chemosensory epithelial cells compared to other epithelial cells. This allows for identification of the fluorescent cholinergic brush cells by FACS. The single-cell solution preparation procedure is based on initial incubation of the snout with dispase solution, which allows for easy mechanical separation of the mucosa from the underlying bones and cartilages. The sample is subsequently incubated with 26 U/ml papain solution for 40 min at 37°C with agitation, which delivers fine dissociation of tight and adherens junctions between epithelial cells. This method grants access to a significant number of viable cells in a single-cell suspension for FACS sorting, RNA-seq, and functional assays.
Materials and Reagents
20, 200, and 1,000 μl Pipette tips (no specific brand)
Thermo ScientificTM screw cap microtubes (2.0 ml) (Fisher Scientific, catalog number: 21-403-202)
3 ml syringes (BD Biosciences, catalog number: 309657)
18 G 1.5 in needles (BD Biosciences, catalog number: 305196)
21 G 1.5 in needles (BD Biosciences, catalog number: 305167)
50 ml conical tubes (Crystalgen, catalog number: 23-2263)
12 × 75 mm (5 ml) round bottom polystyrene tubes (Corning, catalog number: 352052)
Petri dish (Falcon, catalog number: 351029)
Aluminum foil
Dispase powder (Gibco, catalog number: 17105041)
ChATBAC-eGFP mice (B6.Cg-Tg (RP23-268L19-EGFP) 2Mik/J) (The Jackson Laboratory, catalog number: 7902)
200 Proof Ethanol (Koptec, catalog number: V1001)
DNase I (Sigma, catalog number: 10104159001)
Dulbecco’s Phosphate-Buffered Saline (PBS) (Boston BioProducts, catalog number: BSS-220DM-C)
DMEM/F-12 (Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12) (ThermoFisher Scientific, catalog number: 11320033)
Heat-inactivated fetal bovine serum (FBS) (ThermoFisher Scientific, catalog number: 10082139)
Tyrode’s Solution (with bicarbonate, HEPES, and 0.25% BSA, without calcium) (Boston BioProducts, catalog number: PY-912)
Papain from papaya latex (Sigma, catalog number: P3125)
L-cysteine (Sigma, catalog number: C7352)
Tyrode’s Solution (with HEPES and calcium) (Boston BioProducts, catalog number: BSS-355)
Leupeptin trifluoroacetate salt (leupeptin) (Sigma, catalog number: L2023)
HBSS, 1× without calcium, magnesium, and phenol red (Hank’s Balanced Salt Solution) (Corning, catalog number: 21-022-CV)
EDTA (0.5 M), pH 8.0. RNase-free (Thermo Fisher, catalog number: AM9260G)
TruStain FcXTM (anti-mouse CD16/32) antibody (Biolegend, catalog number: 101320)
Pacific Blue anti-mouse CD45 monoclonal antibody (Biolegend, catalog number: 103126)
Allophycocyanin (APC) anti-mouse CD326 (EpCAM) monoclonal antibody (Biolegend, catalog number: 118214)
Propidium iodide (PI) (Sigma, catalog number: P4170)
70% EtOH (500 ml) (see Recipes)
Dispase solution (see Recipes)
DMEM-based stopping solution (see Recipes)
Tyrode buffer-based digesting solution with papain (see Recipes)
Tyrode buffer-based stopping solution (see Recipes)
FACS buffer (washing buffer for flow cytometry) (see Recipes)
Equipment
Dissection scissors (straight) (Fisher Scientific, catalog number: 08-951-5)
Forceps (straight, serrated) (Fisher Scientific, catalog number: 13-812-36)
Pipettes (P10, P200, P1000) (no specific brand)
FisherbrandTM IsotempTM General Purpose Deluxe Water Bath (Fisher Scientific, catalog number: FSGPD05)
FisherbrandTM Multiplatform Shaker (Fisher Scientific, catalog number: 88-861-021)
Dissecting microscope (Leica, catalog number: M165FC)
Scalpel (No.10) (ThermoFisher Scientific, catalog number: 3120032)
Fisher Vortex Genie 2 (Fisher Scientific, catalog number: 12-812)
100 μM FisherbrandTM Sterile Cell Strainers (Fisher Scientific, catalog number: 22-363-549)
Sorvall Legend X1R Centrifuge (ThermoFisher Scientific, catalog number: 75004261)
BD LSRFortessaTM Flow Cytometer (BD Biosciences)
Software
FlowJo v.8 (FlowJo, LLC, https://www.flowjo.com)
Prism 7 (GraphPad Software, https://www.graphpad.com/scientific-software/prism/)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Ualiyeva, S., Boyd, A. A., Barrett, N. A. and Bankova, L. G. (2021). Isolation of Nasal Brush Cells for Single-cell Preparations. Bio-protocol 11(18): e4163. DOI: 10.21769/BioProtoc.4163.
- Ualiyeva, S., Hallen, N., Kanaoka, Y., Ledderose, C., Matsumoto, I., Junger, W. G., Barrett, N. A. and Bankova, L. G. (2020). Airway brush cells generate cysteinyl leukotrienes through the ATP sensor P2Y2. Sci Immunol 5(43).
分类
细胞生物学 > 细胞分离和培养
免疫学 > 动物模型 > 小鼠
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link