- EN - English
- CN - 中文
Identification and Quantitation of Neutrophil Extracellular Traps in Human Tissue Sections
人体组织切片中中性粒细胞胞外陷阱的鉴定与定量
发布: 2021年09月20日第11卷第18期 DOI: 10.21769/BioProtoc.4159 浏览次数: 5415
评审: Pilar Villacampa AlcubierreSarajo MohantaNicoletta Cordani

相关实验方案

在活细胞成像中测量趋化因子刺激下Jurkat细胞的Piezo1和肌动蛋白极性
Chinky Shiu Chen Liu [...] Dipyaman Ganguly
2024年10月05日 1802 阅读
Abstract
Neutrophils are one of the first innate immune cells recruited to tissues during inflammation. An important function of neutrophils relies on their ability to release extracellular structures, known as Neutrophil Extracellular Traps or NETs, into their environment. Detecting such NETs in humans has often proven challenging for both biological fluids and tissues; however, this can be achieved by quantitating NET components (e.g., DNA or granule/histone proteins) or by directly visualizing them by microscopy, respectively. Direct visualization by confocal microscopy is preferably performed on formalin-fixed paraffin-embedded (FFPE) tissue sections stained with a fluorescent DNA dye and antibodies directed against myeloperoxidase (MPO) and citrullinated histone 3 (Cit-H3), two components of NETs, following paraffin removal, antigen retrieval, and permeabilization. NETs are defined as extracellular structures that stain double-positive for MPO and Cit-H3. Here, we propose a novel software-based objective method for NET volume quantitation in tissue sections based on the measurement of the volume of structures exhibiting co-localization of Cit-H3 and MPO outside the cell. Such a technique not only allows the unambiguous identification of NETs in tissue sections but also their quantitation and relationship with surrounding tissues.
Graphic abstract:
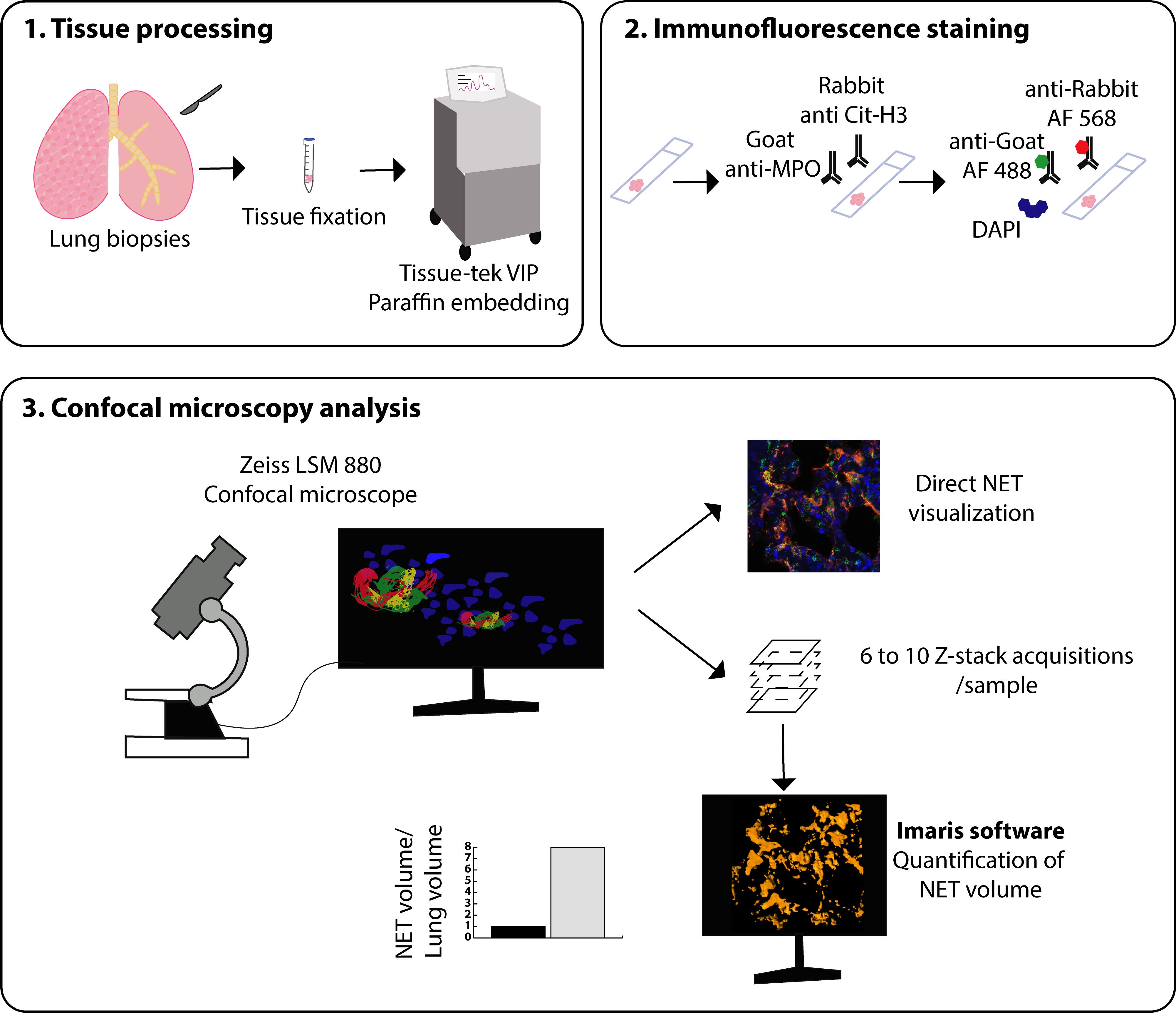
Graphical representation of the methodology used to stain and quantitate NETs in human lung tissue.
Background
Neutrophils are the major innate immune cells in the blood (50-70% of white blood cells in humans) (Mestas and Hughes, 2004). These cells have a diameter of 5-7 µm and are characterized by a segmented nucleus, secretory vesicles in their cytoplasm, and a short lifespan (Borregaard, 2010). Neutrophils are produced in the bone marrow in response to G-CSF secretion under the control of key transcription factors such as PU.1, C/EBPα, GFI-1, and C/EBPϵ (Hidalgo et al., 2019). Neutrophils participate in pathogen destruction by releasing the cytotoxic contents of their secretory vesicles or by phagocytosis (Borregaard, 2010). Recently, several discoveries have shed light on neutrophil biology and function. First, a study using radiolabeling of neutrophils demonstrated an unexpected longer half-life of these cells, which can reach 5.4 days in humans and 18 hours in mice (Pillay et al., 2010). Second, in 2004, Brinkmann and colleagues discovered a novel bactericidal action of neutrophils. Indeed, it was observed that the release of particular structures could trap bacteria and limit their dissemination throughout the organism (Brinkmann et al., 2004). These new structures were named Neutrophil Extracellular Traps or NETs. NETs are web-like structures composed of extracellular free DNA associated with a particular form of citrullinated histone 3 (Cit-H3), in which arginine residues have been replaced by citrulline, and various antimicrobial peptides from secretory vesicles, such as myeloperoxidase (MPO), neutrophil elastase (NE), or cathelicidins (Papayannopoulos, 2018). The release of these structures can be triggered by various stimuli derived from bacteria, viruses, parasites, and fungi, but also by immune complexes, crystals, pro-inflammatory cytokines, and chemokines (Papayannopoulos, 2018). Within neutrophils, the activation of pyruvate kinase C, formation of reactive oxygen species (ROS), and the activation of peptidyl arginase 4 and neutrophil elastase allow chromatin decondensation, nuclear membrane disruption, and NET release (Papayannopoulos, 2018). NETs can immobilize pathogens and prevent their dissemination but are also able to kill them directly (Brinkmann et al., 2004; Papayannopoulos, 2018). Unfortunately, excessive NET release or inappropriate NET accumulation can trigger important tissue damage and lesions (Narasaraju et al., 2011) or inadequate activation of immune cells, thereby promoting various immune-mediated disorders (Hakkim et al., 2010; Radermecker et al., 2019) or coagulation issues (Middleton et al., 2020). Thus, NETs are increasingly implicated in various pathological processes, as evidenced by experimental work in mouse models (Radermecker et al., 2019; Villanueva et al., 2011). The investigation into their potential contributions to human disease is therefore of great interest. NET detection in humans is quite challenging and can be performed either indirectly in physiological fluids or directly in tissue sections. To date, NETs have been mainly measured in physiological fluids such as serum and bronchoalveolar lavage fluid (BALF) (Toussaint et al., 2017; Middleton et al., 2020). NET measurements in fluids rely on the detection of one or two of their components (extracellular DNA, citrullinated histone 3, or complexes of DNA/MPO or DNA/NE). Extracellular free DNA can be detected using DNA quantitation assays on serum or BALF supernatant (Toussaint et al., 2017). Methods for the detection of Cit-H3 have been developed, including western blotting (Liu et al., 2016; Thålin et al., 2017). A more specific technique relies on the detection of complexes formed by DNA and one of the antimicrobial peptides released from secretory vesicles, like MPO or NE, by ELISA (Caudrillier et al., 2012; Caldarone et al., 2019). These techniques are rapid but lack specificity and do not provide any information about the location of NETs in the tissue. Here, we describe a protocol allowing the direct visualization and quantitation of NETs in situ by confocal microscopy. NETs are identified as extracellular structures exhibiting a co-localization of Cit-H3 and MPO, two important components of NETs. This technique is currently considered the gold standard of NET detection. Furthermore, we propose a novel software-based objective method to quantitate NETs in tissues based on the measurement of the volume of structures exhibiting a co-localization of Cit-H3 and MPO outside the cell (Radermecker et al., 2019). This technique is specific, quantitative, and provides information about the location of NETs in tissues (Radermecker et al., 2020). Identification of specific NET-rich areas in human tissues may promote a better understanding of their pathological roles in disease.
Materials and Reagents
Coverslips, 22 × 22 mm (Fisher Scientific, ThermoFisher Scientific, catalog number: 15727582)
Microscope slides, Superfrost (VWR, catalog number: 631-0113)
Pipette tips
Bovine serum albumin (Sigma-Aldrich, catalog number: 9048-46-8), stored at 4-8°C
Donkey serum (Sigma-Aldrich, catalog number: D9663), stored at -20°C
Goat anti-human/mouse myeloperoxidase antibody (Novus Biologicals, catalog number: AF3667), stored at -20°C
Rabbit anti-histone H3 (citrulline R2+R8+R17) antibody (Abcam, catalog number: ab5103), stored at -20°C
Donkey anti-goat IgG (H+L) highly cross-adsorbed secondary antibody, Alexa Fluor 488-conjugated (ThermoFisher Scientific, catalog number: A32814), stored at 4-8°C
Donkey anti-rabbit IgG (H+L) highly cross-adsorbed secondary antibody, Alexa Fluor 568-conjugated (ThermoFisher Scientific, catalog number: A10042), stored at 4-8°C
Neo Clear (Sigma-Aldrich, catalog number: 64741-65-7)
Absolute ethanol (Fisher Chemical, catalog number: E/0600DF/15)
Xylene (VWR, catalog number: 28973.363)
Ultrapure DNase/RNase-free distilled water (ThermoFisher Scientific, Invitrogen, catalog number: 10977049)
DAPI (Sigma-Aldrich, catalog number: 28718-90-3), stored at -20°C
ProLong Gold antifade mountant (ThermoFisher Scientific, catalog number: P10144), stored at -20°C
Goat IgG isotype control (ThermoFisher Scientific, catalog number: 31245), stored at 4-8°C
Rabbit IgG isotype control (ThermoFisher Scientific, catalog number: 31235), stored at 4-8°C
Formaldehyde (Sigma-Aldrich, catalog number: 47608)
HIER Tris-EDTA Buffer, pH 9.0 (10×) (Zytomed Systems, catalog number: ZUC029-500), stored at 4-8°C
Tween-20 (Fisher Scientific, Acros Organics, catalog number: 10491081), stored at room temperature (RT)
Triton X-100 (Sigma-Aldrich, catalog number: 9002-93-1), stored at room temperature (RT)
Antigen Retrieval Buffer (see Recipes)
Permeabilization Buffer (see Recipes)
Blocking Buffer (see Recipes)
Equipment
Tissue-Tek-VIP 5 (Sakura)
Rotary microtome (Leica)
Zeiss LSM 880 Airyscan Elyra s.1. confocal microscope (Zeiss)
StainTray slide staining system (Sigma-Aldrich, catalog number: Z670146)
Kartell PMPHellendhal staining jar (ThermoFisher Scientific, Fisher Scientific, catalog number: 10375681)
Portable steam sterilizer (Prestige Medical, 2100 classic)
Pipettes
Refrigerator (4°C)
Freezer (-20°C)
Chemical fume hood
Software
ImageJ (version 15.1n, NIH, https://imagej.nih.gov/ij/download.html)
Imaris (version 7.5.2, oxford instruments)
Zen black (Zeiss)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Radermecker, C., Hego, A., Delvenne, P. and Marichal, T. (2021). Identification and Quantitation of Neutrophil Extracellular Traps in Human Tissue Sections. Bio-protocol 11(18): e4159. DOI: 10.21769/BioProtoc.4159.
分类
免疫学 > 免疫细胞染色 > 免疫检测
免疫学 > 免疫细胞成像 > 共聚焦显微镜技术
细胞生物学 > 组织分析 > 组织染色
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link











