- EN - English
- CN - 中文
A Co-purification Method for Efficient Production and Src Kinase-mediated Phosphorylation of Aplysia Cortactin
一种高效生产和Src激酶介导的马海兔磷酸化的共纯化方法
发布: 2021年09月20日第11卷第18期 DOI: 10.21769/BioProtoc.4158 浏览次数: 3630
评审: David PaulDeepali BhandariSeda Ekici
Abstract
Cortactin is an actin-binding protein that regulates processes like cell migration, endocytosis, and tumor cell metastasis. Although cortactin is associated with actin-cytoskeletal dynamics in non-neuronal cells and cell-free systems, the exact mechanisms underlying its fundamental roles in neuronal growth cones are not fully explored. Recent reports show that Aplysia Src2 tyrosine kinase induces phosphorylation of cortactin as a mechanism to control lamellipodia protrusion and filopodia formation in cultured Aplysia bag cell neurons (He et al., 2015; Ren et al., 2019). In order to provide in vitro evidence for Src2-mediated phosphorylation of cortactin, we developed a robust and cost-effective method for the efficient expression and purification of Aplysia cortactin and Src2 kinase that can be used for biochemical studies including phosphorylation assays. By co-purifying cortactin and Src kinase with a phosphatase (YopH) from Yersinia enterocolitica, we eliminated the problem of non-specific phosphorylation of induced proteins by bacterial kinases and also reduced costs by bypassing the need for commercial enzymatic treatments. This protocol is reproducible and can be modified to produce homogenous non-phosphorylated proteins during recombinant protein expression in Escherichia coli.
Keywords: Cortactin (皮质素)Background
An organism’s nervous system is highly dynamic and enables cognition, growth, breathing, physical and emotional sensation, and other daily activities. The nervous system is comprised of a vast network of specialized cells, called neurons, that work together to transmit signals between different parts of the body (Abbasi et al., 2018). Each neuron has a cell body and extensions of different lengths. Shorter extensions, called dendrites, receive and transmit signals to the neuronal cell body (Lovinger, 2008; Reference 20). Longer extensions, called axons, carry electrical impulses away from the cell body to muscles, glands, and distant neurons (Lovinger, 2008). At the cellular level, nervous system function relies on precise wiring of these neurons, breaches to which lead to neurological disorders (Goodman and Shatz, 1993; Chédotal and Richards, 2010; Engle, 2010; Kolodkin and Tessier-Lavigne, 2011; Ren et al., 2019). Specialized extensions located at the tips of dendrites and axons, called neuronal growth cones, play a key role in ensuring proper axonal growth and guidance to establish functional connectivity (Lowery and Vactor, 2009; Vitriol and Zheng, 2012). Neuronal growth cones are highly motile sensory units that can detect extracellular cues and convert them into intracellular signals (Suter and Forscher, 2000; Dent et al., 2011). Growth cones use these signals to remodel cytoskeletal proteins, including F-actin, which constitute structures like lamellipodia and filopodia that are needed for motility. Thus, growth cones control axonal growth and guidance and the formation of neuronal circuitry. Therefore, elucidating the signaling mechanisms that dictate actin organization and dynamics during growth cone motility is imperative to understand the process of nervous system development and regeneration.
Cortactin is an actin-binding protein known to regulate cytoskeletal dynamics and cell migration in non-neuronal cells. Cortactin consists of an amino terminal acidic domain (NTA), followed by an F-actin-binding domain, an alpha-helical domain, a proline-rich region (PRR), and an SH3 domain at the C-terminus (Figure 1) (Ammer and Weed, 2008; Decourt et al., 2009). It was first characterized as a substrate for Src kinase and regulates several actin-related processes, including cell migration, tumor metastasis, and response to pathogens (Schnoor et al., 2018). Cortactin is important in the assembly, branching, and stabilization of cytoskeletal structures, such as lamellipodia and filopodia (Helgeson and Nolen, 2013; He et al., 2015). While cortactin is known to undergo multiple post-translational modifications, including phosphorylation (Schnoor et al., 2018; Ren et al., 2019) and acetylation, not much is known about the exact mechanisms underlying its fundamental roles in neuronal growth cones and filopodia formation in neurons.
Using cultured Aplysia bag cell neurons as a model, recent findings demonstrated that phosphorylation of a single tyrosine 499 residue of cortactin mediated by the Src2 kinase is important for the formation of filopodia and the regulation of actin organization and dynamics in growth cones (He et al., 2015; Ren et al., 2019). We have also demonstrated with purified proteins that Aplysia Src2 phosphorylates Aplysia cortactin in vitro (Ren et al., 2019). Here, we describe in detail the robust and cost-effective assay to assess the direct phosphorylation of cortactin by Src2 kinase used in our previous study (Ren et al., 2019). Using E. coli (BL21-DE3) cells as a host, we developed a reproducible two-step protocol to produce pure cortactin and Src kinase proteins at high yields.
Src kinases are phosphoryl transferases that transfer the γ-phosphate of ATP to tyrosine residues on specific substrate proteins. A common method used to detect kinase activity is a radioactive ATP kinase assay (Karra et al., 2017). This kinase assay protocol tracks the transfer of the radio-isotope 32P from ATP [γ-32P] to the substrate. Incorporation of this radiolabeled phosphate into the kinase substrate is then measured using autoradiography. Despite the sensitivity of this assay using optimized protocols with human Src and cortactin (Tehrani et al., 2007), we were unable to detect phosphorylation of Aplysia cortactin by Src kinase in vitro. Because bacteria also possess kinases, we reasoned and found to be true that our bacterially expressed and purified Src and cortactin proteins were non-specifically phosphorylated by bacterial kinases (Shrestha et al., 2012) during the purification process, therefore preventing Src2-mediated phosphorylation of cortactin. We circumvented these issues by co-purifying Aplysia Src2 and cortactin in the presence of a phosphatase called YopH (encoded by Yersinia enterocolytica) (Zhang et al., 1992), followed by sequential affinity and size exclusion chromatography, which yielded pure, unphosphosphorylated proteins that could be used in an in vitro kinase assay. By incorporating YopH into the purification scheme, we removed residual phosphate groups from the proteins that may have been introduced during the heterologous expression step. Here, we describe the co-purification process and present data obtained using this method, revealing the first direct biochemical evidence that Aplysia Src2 phosphorylates Aplysia cortactin and that Y505 (Cort-FYF) is preferentially modified in vitro, whereas Y499 is the essential tyrosine that is phosphorylated by Src2 in neuronal growth cones (see Figure 6). This procedure could be more generally adopted to remove non-specific phosphorylation of other proteins expressed in a prokaryotic host.
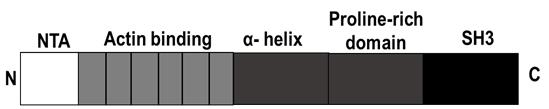
Figure 1. Schematic representation of the functional domain structure of Cortactin (human). N = N terminus, C = C-terminus, NTA = amino-terminal acidic region, SH3 = Src homology 3 domain. Schematics were created with Biorender.com.
Materials and Reagents
pSMT3 plasmid (Sanyal et al., 2015)
Ampicillin (AMRESCO, catalog number: 0339-100G)
Kanamycin (Fisher Bioreagents, catalog number: BP906-5)
Isopropyl p-Dthiogalactoside (IPTG) (Fisher Bioreagents, catalog number: BP1755-10)
Liquid Broth (LB) (Fisher Bioreagents, catalog number: BP1426500 )
Glycerol (Fisher Reagents, catalog number: BP229-4)
Phenylmethylsulfonyl fluoride (PMSF) (Sigma-Aldrich, catalog number: p7626-25g)
Amicon ultra-4 centrifugal filter unit (Sigma-Aldrich, catalog number: UFC 805024)
Liquid nitrogen
Escherichia coli (E. coli) BL21 (DE3) (Strategene, catalog number: 200131)
10% Mini-PROTEAN® TGXTM Precast Protein Gels (Bio-Rad Laboratories, catalog number: 456-1033)
Adenosine -5′-triphosphate [γ-32P] (Perkin-Elmer)
Coomassie Stain (AMRESCO, catalog number: 0472-25G)
Dithiothreitol (DTT) (Alfa Aesar, catalog number: A15797)
Sodium chloride (NaCl) (Fisher Scientific, catalog number: S271-10)
β-Mercaptoethanol (Sigma-Aldrich, catalog number: M3148-250ML)
Tris (Thermo Fisher Scientific, catalog number: BP152-5)
Imidazole (Sigma-Aldrich, catalog number: I2399-500G)
Eppendorf Microcentrifuge (Fisherbrand, catalog number: Q5-408-129)
Nitrocellulose membrane (Thermo Fisher Scientific, catalog number: 88018)
Monoclonal anti-polyhistidine antibody (Sigma-Aldrich, catalog number: H1029-5ML)
Triton X-100 (Sigma-Aldrich, catalog number: T9284-500ML)
Agarose (AMRESCO, catalog number: N605-500G)
Bacto agar (Beckmon, Dickson and Company, catalog number: 214010)
Magnesium chloride hexahydrate (Alfa Aesar, catalog number: 12288)
Manganese chloride tetrahydrate (Sigma-Aldrich, catalog number: M3634-500G)
Sodium orthovanadate (MP Biomedicals, catalog number: 159664)
[γ-32P] radioactive ATP (Perkin-Elmer, catalog number: BLU502A100UC)
Bovine serum albumin (Fisher Bioreagents, catalog number: BP1605-100)
Tween 20 ( Bio-Rad Laboratories, catalog number: 1706531)
IRDye® 680LT Goat anti-Mouse IgG Secondary Antibody (P/N: 926-68020)
Lambda protein phosphatase (New England Biolabs, catalog number: P0753S)
Recombinant proteins
WT Aplysia cortactin and single tyrosine mutants were subcloned as full-length N-terminal His6-SUMO fusion into pSMT3 expression vector using restriction enzyme cloning (Ren et al., 2019).
Note: Cortactin mutant proteins are indicated as YFF, FYF, and FFY where tyrosine was mutated to phenylalanine to test which of the three putative tyrosine residue (Y499, Y505, and Y509), respectively, in Aplysia cortactin is the preferred phosphorylation sites in vitro versus what was previously observed in vivo in neuronal growth cones. FFF is the triple tyrosine phosphorylation-defective mutant.
Src2 kinase (Src2) and the enzymatically inactive dominant negative Src2 (DNSrc2) cloned into pET-Duet with an N-terminal His6-tag (Ren et al., 2019).
GST-tagged, truncated YopH phosphatase missing 184 residues at the N-terminal and with an Arg in place of Ser at position 235 from Yersinia enterocolitica cloned in the plasmid pT7-7 (YopH) (Zhang et al.,1992); provided by Jack Dixon, University of California, San Diego.
Note: All plasmids are available upon request from the corresponding author.
Protein sample buffer for SDS-PAGE (made in house; see Recipes)
Liquid LB media
Solid LB plate
Stock preparation (see Recipes)
1 M IPTG
1,000× kanamycin stock
1,000× ampicillin stock
Buffer Preparation (see Recipes)
Lysis Buffer
Wash Buffer
Elution Buffer
SEC Buffer
Kinase Buffer
Sample loading buffer
10× Transfer Buffer
1× Tris-Buffered Saline, 0.1% Tween® 20 Detergent (TBST)
5% BSA
1× Transfer Buffer
Equipment
Note: Alternate yet equivalent equipment can be used.
4-L Erlenmeyer flask (Avantor VWR, catalog number: 10545-846)
UV/VIS spectrophotometer (Beckman Coulter, model: DU 350)
Refrigerated benchtop centrifuge (Beckman Coulter, model: ALLEGRA X-14R)
ÄKTA Purifier chromatography system (GE Healthcare, model: ÄKTA Pure)
High-speed centrifuge (Beckman Coulter, model: Avanti J-E)
Rotor capable of spinning 250 ml bottles (Nalgene, catalog number: 3120-1000)
Shaker incubator (New Brunswick, model: Innova®43) or an equivalent incubator that can be set at 37°C
Ice-water bath (VWR International, model: 1225PC)
Pipetman (Rainin)
Hiload 16/600 Superdex 200 PG column (GE Healthcare, catalog number: 28-9893-35)
PCR machine (Thermo Fisher Scientific, model: Mastercycler)
DNA agarose gel imaging equipment (UV transilluminator)
Sonicator (Branson Sonic Power Co., model: cell disruptor 350)
Odyssey imaging system (LI-COR, Biosciences, Lincoln, NE)
SDS-PAGE apparatus with power supply (Bio-Rad Laboratories)
Microwave
Bunsen burner
Typhoon phosphorimager (FLA 9500, GE Healthcare)
ImageQuant TL software (GE Healthcare)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Brown, S. L., Ren, Y., Suter, D. M. and Mattoo, S. (2021). A Co-purification Method for Efficient Production and Src Kinase-mediated Phosphorylation of Aplysia Cortactin. Bio-protocol 11(18): e4158. DOI: 10.21769/BioProtoc.4158.
分类
神经科学 > 细胞机理 > 蛋白质分离
生物化学 > 蛋白质 > 翻译后修饰
生物科学 > 生物技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link