- EN - English
- CN - 中文
Cortical Laminar Recording of Multi-unit Response to Distal Forelimb Electrical Stimulation in Rats
大鼠前肢远端电刺激的多单位反应的皮层层析记录
发布: 2021年11月20日第11卷第22期 DOI: 10.21769/BioProtoc.4153 浏览次数: 3337
评审: Oneil Girish BhalalaZijian ZhangAndrew L. EagleAnonymous reviewer(s)

相关实验方案
基于 rAAV-α-Syn 与 α-Syn 预成纤维共同构建的帕金森病一体化小鼠模型
Santhosh Kumar Subramanya [...] Poonam Thakur
2025年12月05日 1583 阅读
Abstract
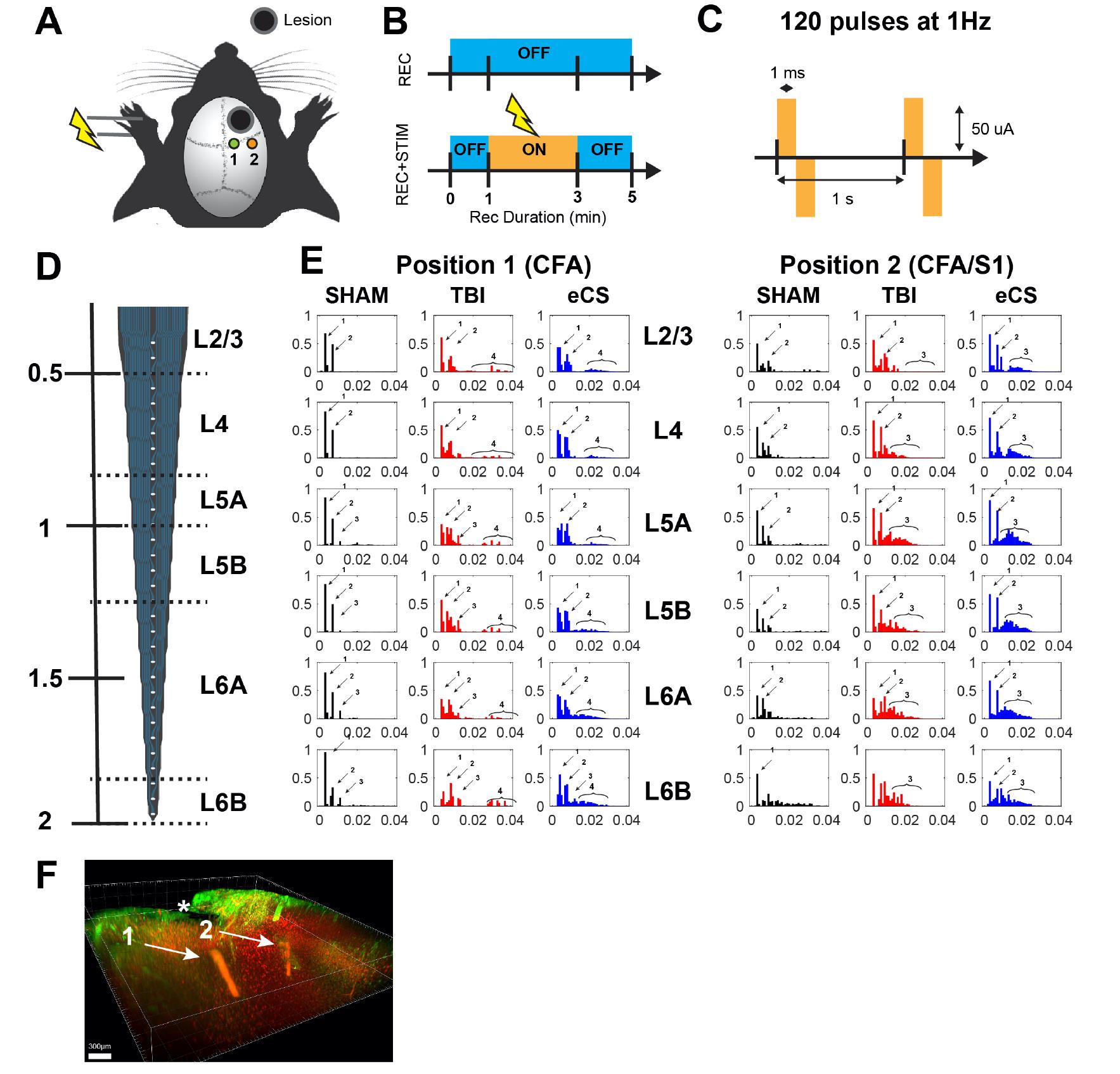
Severe traumatic brain injury (sTBI) survivors experience permanent functional disabilities due to significant volume loss and the brain’s poor capacity to regenerate. Chondroitin sulfate glycosaminoglycans (CS-GAGs) are key regulators of growth factor signaling and neural stem cell homeostasis in the brain. In this protocol, we describe how to perform recordings to quantify the neuroprotective and regenerative effect of implanted engineered CS-GAG hydrogel (eCS) on brain tissue. This experiment was performed in rats under three conditions: healthy without injury (Sham), controlled cortical impact (CCI) injury on the rostral forelimb area (RFA), and CCI-RFA with eCS implants. This protocol describes the procedure used to perform the craniotomy, the positioning of the cortical recording electrode, the positioning of the stimulation electrode (contralateral paw), and the recording procedure. In addition, a description of the exact electrical setup is provided. This protocol details the recordings in the brain of injured animals while preserving most of the uninjured tissue intact, with additional considerations for intralesional and laminar recordings of multi-unit response.
Graphic abstract:
Sensorimotor response to paw stimulation using cortical laminar recordings.
Background
Traumatic brain injury (TBI) is a common and increasingly prevalent problem that affects approximately 69 million people globally, without an effective treatment to date (Dewan et al., 2018). Given the failure of secondary neuroprotective strategies, such as decompressive craniotomy, or tight blood pressure regulation, in ameliorating poor functional outcomes, increased attention has turned to re-establishing damaged neuronal circuitry in the brain through biomaterial implants, with or without concurrent cell transplantation (Tan et al., 2020). Apart from providing the ability to inject any one of several natural or synthetic biomaterials into an injury lesion, it is unclear what functional effect these implants have on restoring native neuronal circuits and on higher-order cognitive and motor outcomes. In our manuscript (Latchoumane et al., 2021, DOI: 10.1126/sciadv.abe0207), we implanted rats with engineered chondroitin-sulfate glycosaminoglycans (eCS) as a potential treatment for the loss of tissue and consequent loss of motor function following TBI. To assess the physiological recovery promoted by eCS implants, we recorded the laminar cortical activity in response to electrical stimulation of the paw in anesthetized rats. Previous works in the field have used ex vivo planar multielectrode arrays on 300 µm brain slices to evaluate field excitatory postsynaptic potentials (fEPSPs) post-biomaterial implant (Yang et al., 2015; Hao et al., 2017). Other labs have performed steady-state evoked potentials (SSEP) recordings with chronically implanted electrodes in the mouse brain to measure brain responses post-implant (Fernández-García et al., 2016). We present a simple protocol that can be carried out in a single procedure, using a multichannel system recording and stimulation setup for the evaluation of the sensorimotor integration in animals implanted with eCS following TBI. Our protocol demonstrates the feasibility and reproducibility of recording perilesionnally to assess biomaterial integration, the impact of eCS on surrounding tissue, and the extent of live neuronal proliferation within and around the implant.
Materials and Reagents
Suture 4-0 Ethicon Absorbable plus antibacterial (Vicryl, catalog number: 109162)
Self tapping screws (18-8 Stainless Steel Slotted Flat Head Screws, M0.8 × 0.2 mm Thread, 2 mm Long; McMastercarr, catalog number: 91430A143)
Sterile cotton swabs, Cotton Tipped Applicators 6"/Sterile 100/box (Dynarex)
Stimulation needles, sterile stainless steel needle 24 gauge (BD, Microlance, catalog number: 1730738)
Animals: Sprague-Dawley Rats, male, age (7-10 weeks) (Charles River, catalog number: 400)
32 channel linear probe (Neuronexus, A1x32-6mm-50-177-CM32, 15 µm thickness, Gen4, lot# P994)
Ketamine 100 mg/ml (Coventrus, catalog number: 056347361-4)
Xylazine cocktail, 100mg/ml (Sigma-Aldrich, catalog number: 7361-61-7)
Isofluorane (Coventrus, catalog number: 029405 )
Buprenorphine, 0.03 mg/ml (Coventrus, catalog number: 059122)
Marcaine, 0.5% (Coventrus, catalog number: 054893)
Povidone-Iodine, 10% topical solution (CVS, catalog number: 59779-085)
Etch-Gel, phosphoric acid 40% (DMG, catalog number: 61901)
Gel Foam® (Pfizer, catalog number: 115631)
SeaKem® agarose (Lonza, catalog number: 50004)
Triple antibiotics, 0.9 g Pouch (25 ct. Box) (Safetec, catalog number: 53205)
Ketamine/Xylazine Cocktail (see Recipes)
Ketamine/Xylazine Cocktail:
For 1 ml solution: 0.9 ml of Ketamine (100 mg/ml) + 0.1 ml of Xylazine (100 mg/ml)
KX rat cocktail 0.1 ml/100g rat wt. IP (Ketamine: 90 mg/kg, Xylazine: 9.0 mg/kg)
Equipment
Multichannel acquisition system (MCS, Wireless Recording, model: W2100)
Multichannel recording and stimulation headstage (MCS, headstage, model: HS32-EXT0.5mA)
Dell PC i7, RAM:8Go, SSD: 500 GoPremium Silicone
Kopf Stereotaxic frame for rodent with manipulating arm
Electronic breadboard (Half-size breadboard; 63; adafruit.com)
Electronic cables (Covered Male-Male Jumper Wires, 200 mm × 40; Adafruit.com, catalog number: 4482)
MCS coaxial TTL cable (C-BNC-Lemo1m; Multichannel system accessories)
Electric Shaver (Philips, Norelco oneblade QP2520/90)
Electric Drill and trephine bur (Micromotor shiyang, H102S)
CCI tip, 3 mm diameter (Custom made)
CCI impactor machine (UGA workshop custom made)
Software
Acquisition Software: MCS experimenter (Multichannel Systems, https://www.multichannelsystems.com/)
System configuration: MCS IPconfig (Multichannel Systems, https://www.multichannelsystems.com/)
Data analysis: Matlab + MCS toolbox (Multichannel Systems, https://www.multichannelsystems.com/)
Matlab R2019b (Mathworks Inc., mathworks.com)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Latchoumane, C. V., Forghani, R. and Karumbaiah, L. (2021). Cortical Laminar Recording of Multi-unit Response to Distal Forelimb Electrical Stimulation in Rats. Bio-protocol 11(22): e4153. DOI: 10.21769/BioProtoc.4153.
- Latchoumane, C. V., Betancur, M. I., Simchick, G. A., Sun, M. K., Forghani, R., Lenear, C. E., Ahmed, A., Mohankumar, R., Balaji, N. and Mason, H. D. (2021). Engineered glycomaterial implants orchestrate large-scale functional repair of brain tissue chronically after severe traumatic brain injury. Sci Adv 7(10): eabe0207.
分类
生物物理学 > 电生理
神经科学 > 神经系统疾病 > 动物模型
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link