- EN - English
- CN - 中文
Kinetic Analysis of a Protein-protein Complex to Determine its Dissociation Constant (KD) and the Effective Concentration (EC50) of an Interplaying Effector Molecule Using Bio-layer Interferometry
蛋白质-蛋白质复合物的动力学分析,以确定其电离常数 (KD) 和使用生物层干涉法相互作用的效应分子的有效浓度 (EC50)
发布: 2021年09月05日第11卷第17期 DOI: 10.21769/BioProtoc.4152 浏览次数: 6012
评审: Manjula MummadisettiVicente RubioAnonymous reviewer(s)
Abstract
Biolayer interferometry (BLI) is an emerging analytical tool that allows the study of protein complexes in real time to determine protein complex kinetic parameters. This article describes a protocol to determine the KD of a protein complex using a 6×His tagged fusion protein as bait immobilized on the NTA sensor chip of the FortéBio® Octet K2 System (Sartorius). We also describe how to determine the half maximal effective concentration (EC50, also known as IC50 for inhibiting effectors) of a metabolite. The complete protocol allows the determination of protein complex KD and small molecular effector EC50 within 8 h, measured in triplicates.
Graphic abstract:
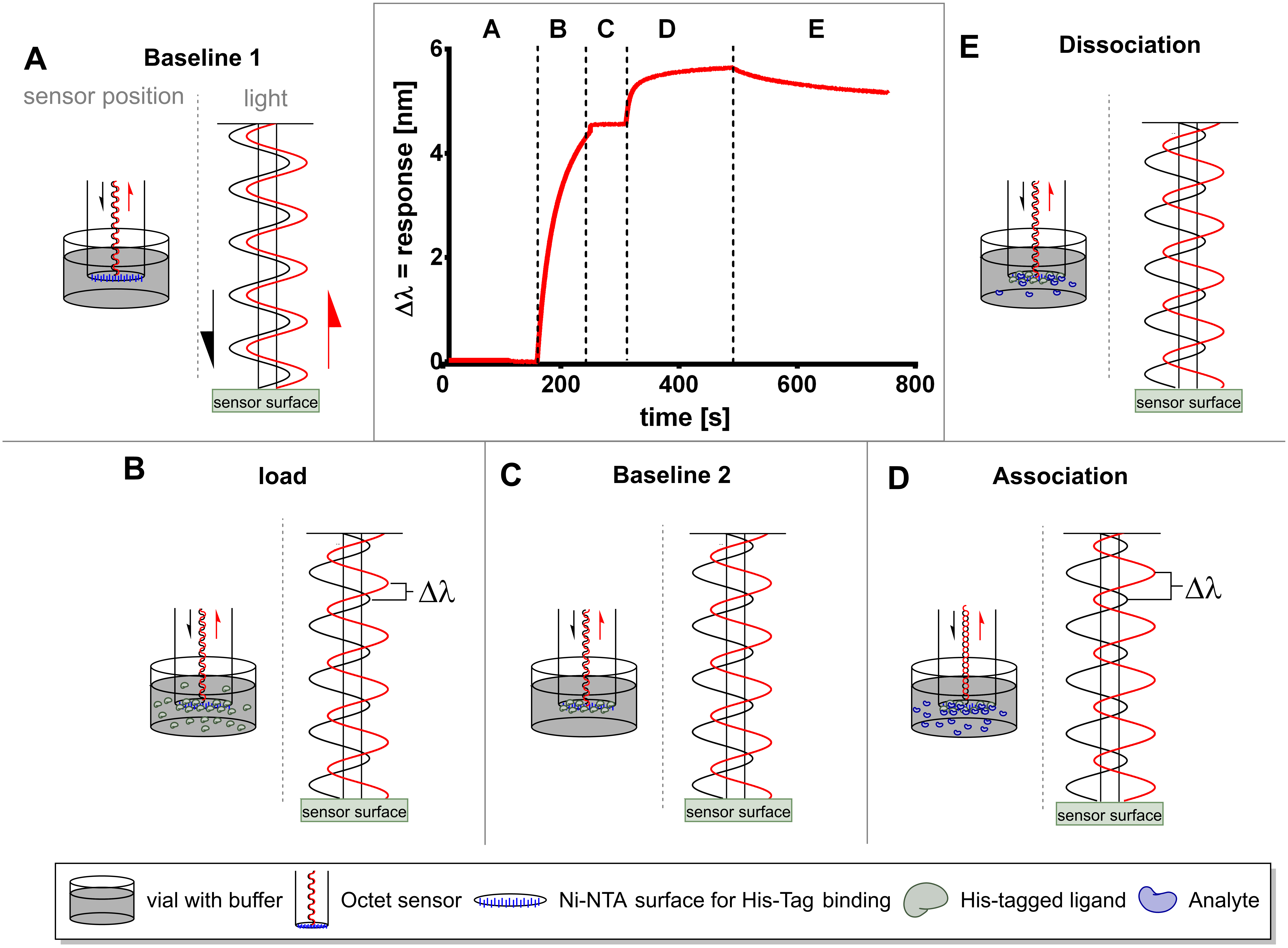
Principle of the Biolayer interferometry measurement. (Middle, top) Exemplary result of the BLI measurement using Octet® (Raw Data). Wavelength shift (Δλ) against time. (A) Baseline 1. Baseline measurement. When the sensor is equilibrated in the kinetics buffer. The light is reflected with no difference. (B) Load. The his-tagged proteins (ligand) are loaded onto the sensor surface. The light is reflected with a shift of the wavelength. (C) Baseline 2. The loaded sensor is equilibrated in the kinetics buffer. No further wavelength shift appears. (D) Association. The loaded sensor is dipped into the analyte solution. The analyte binds to the immobilized ligand along with an increased wavelength shift. (E) Dissociation. Afterward, the sensor is dipped again into the kinetics buffer without the analyte. Some analyte molecules dissociate. The wavelength shift decreases. (Subfigures A-E) The left side shows the position of the sensor during the measurement seen in the representative BLI measurement, marked with the figure label. The right side shows the light path in the sensor. Black waves represent the light emitted to the sensor surface. The red waves show the light reflected from the sensor surface back to the detector.
Background
The Investigation of protein-protein interactions is an important field in life sciences since protein interactions play crucial roles in living organisms. Many biochemical and physiological reactions are under the control of proteins that require physical communication to other proteins within the cell. For example, human cell invasion by SARS-CoV-2 starts with interactions between the viral Spike protein and the human ACE2 receptor (Shang et al., 2020). Determination of the kinetic and affinity parameters of specific protein complexes provides important information about their properties and helps to discover their function and regulatory properties. Furthermore, the determination of the half maximal effective concentration (EC50) of a metabolite, which inhibits or enhances protein complex formation, is crucial for the understanding of regulatory mechanisms and/or the development of drugs to combat infectious diseases.
Several methods are available to study protein interactions in real time, such as biolayer interferometry (BLI), surface plasmon resonance (SPR), or isothermal titration calorimetry (ITC). BLI is an emergent optical analytic technique that provides a good trade-off between sensitivity, cost intensity, reproducibility, and the ability for high-throughput measurements. The physics of BLI is based on the principle of a wavelength shift when white light is reflected from two layers, first from an internal reference layer and second from a protein coated layer (FortéBio, 2005-2006). The wavelength shift is detected by the machine and is recorded over time. The length of the shift is proportional to the bound proteins on the layer and can be used to calculate the binding affinities and kinetics. Compared to ITC, BLI measurement requires lower amounts of the target proteins, which is especially important for proteins that are difficult to express and purify, e.g., membrane proteins. BLI has lower sensitivity compared to SPR methods; however, in terms of equipment maintenance, BLI is advantageous compared to the micro-fluidic SPR systems, which are prone to clogging and require high sample purity, buffer degassing, and constant maintenance of the sensor chips and micro-fluidic devices. In addition, BLI is tolerant to the use of analytes in complex matrices (Shah and Duncan, 2014), enabling high-throughput measurements of crude analyte preparations.
Recently, we employed BLI to characterize the interaction between the PII signaling proteins and different binding target proteins, including NAD synthetase (NadE), phosphoenolpyruvate carboxylase (PEPC), PII-interacting regulator of arginine synthesis (PirA), and PII-interacting regulator of carbon metabolism (PirC) (Santos et al., 2020; Scholl et al., 2020; Bolay et al., 2021; Orthwein et al., 2021). PII proteins sense the cellular energy state through the competitive binding of ATP and ADP, and sense carbon/nitrogen balance through binding of the citric acid cycle metabolite 2‐oxoglutarate (2-OG) (Forchhammer and Selim, 2020; Selim et al., 2020). Here, we describe a robust, simple, and quick protocol for the detection of protein interactions using a His-tagged bait protein (ligand) and a non-His-tagged prey protein (analyte). The BLI measurements were performed using a FortéBio® Octet K2 system (Sartorius). The protocol describes the procedure to determine the dissociation constant (KD) of the protein complex and also the EC50 of small effector molecules (2-OG here), which can promote or disrupt the protein complex. The protocol is exemplified using the PII protein from the unicellular cyanobacterium Synechocystis sp PCC 6803 for studying PII-PirC interactions. In cyanobacteria, the interaction between PII-PirC regulates the central carbon metabolism in response to the intracellular concentrations of the carbon/nitrogen indicator 2-OG (Orthwein et al., 2021).
Materials and Reagents
Microplate, 96-well plate, PS, F-Bottom (Chimney well) Black, Non-Binding (Greiner Bio-One, catalog number: 655900)
HEPES (Thermo Scientific, Fischer Bioreagents, catalog number: BP-310)
KOH (Merck, Millipore, catalog number: 1.05033)
Nonident-P40 (Thermo Fischer Scientific, Fluka, catalog number: 74358)
KCl (Carl Roth, catalog number: 6781.1)
MgCl2·6H2O (Merck, Millipore, catalog number: 7791-18-6)
ATP (store at -20°C) (Carl Roth, catalog number: K054.3)
α-ketoglutaric acid disodium salt hydrate (store at 4°C) (Thermo Fischer Scientific, Fluka, catalog number: K3752) – another name for 2‐oxoglutarate (2-OG)
Glycine (Merck, Sigma-Aldrich, catalog number: 33226)
NiCl2·6H2O (Carl Roth, catalog number: 4489.1)
Kinetics buffer (see Recipes)
Equipment
Ni-NTA (NTA) Dip and ReadTM Biosensors (tray) (Sartorius, FortéBio®, catalog number: 18-5103)
FortéBio® Octet K2 System (Sartorius)
Software
Data Acquisition 11.0.0.64 (FortéBio®, https://www.sartorius.com/en/products/protein-analysis/octet-systems-software)
Data Analysis HT 11.0.0.50 (FortéBio®, https://www.sartorius.com/en/products/protein-analysis/octet-systems-software)
Prism, Version 6.01 or higher (GraphPad Software Inc.)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Orthwein, T., Huergo, L. F., Forchhammer, K. and Selim, K. A. (2021). Kinetic Analysis of a Protein-protein Complex to Determine its Dissociation Constant (KD) and the Effective Concentration (EC50) of an Interplaying Effector Molecule Using Bio-layer Interferometry. Bio-protocol 11(17): e4152. DOI: 10.21769/BioProtoc.4152.
- Santos, A. R. S., Gerhardt, E. C. M., Parize, E., Pedrosa, F. O., Steffens, M. B. R., Chubatsu, L. S., Souza, E. M., Passaglia, L. M. P., Sant'Anna, F. H., de Souza, G. A., Huergo, L. F. and Forchhammer, K. (2020). NAD+ biosynthesis in bacteria is controlled by global carbon/nitrogen levels via PII signaling. J Biol Chem 295(18): 6165-6176.
分类
生物化学 > 脂质 > 膜脂
分子生物学 > 蛋白质 > 蛋白质-蛋白质相互作用
生物科学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link