- EN - English
- CN - 中文
Isolation and Characterization of Membrane Vesicles from Lactobacillus Species
乳酸杆菌属膜囊泡中的分离和特性研究
发布: 2021年09月05日第11卷第17期 DOI: 10.21769/BioProtoc.4145 浏览次数: 4927
评审: Alba BlesaJing LiChenchen Liu
Abstract
Throughout their life cycle, bacteria shed portions of their outermost membrane comprised of proteins, lipids, and a diversity of other biomolecules. These biological nanoparticles have been shown to have a range of highly diverse biological activities, including pathogenesis, community regulation, and cellular defense (among others). In recent publications, we have isolated and characterized membrane vesicles (MVs) from several species of Lactobacilli, microbes classified as commensals within the human gut microbiome (Dean et al., 2019 and 2020). With increasing scientific understanding of host-microbe interactions, the gut-brain axis, and tailored probiotics for therapeutic or performance increasing applications, the protocols described herein will be useful to researchers developing new strategies for gut community engineering or the targeted delivery of bio-active molecules.
Graphic abstract:
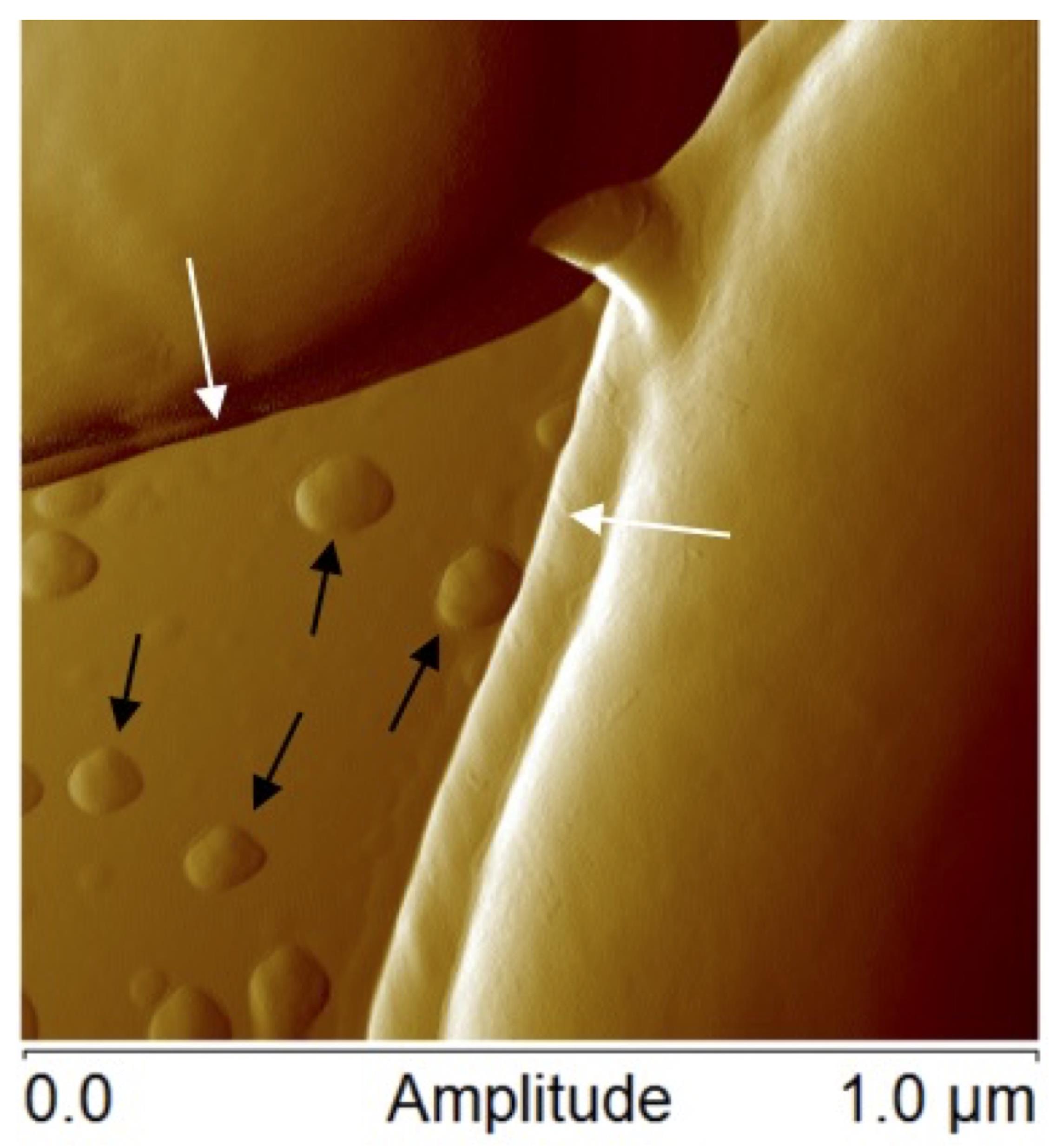
Figure 1. Atomic force microscopic image of Lactobacillus casei ATCC 393 bacteria margins (white arrows) and membrane vesicles (black arrows)
Background
The membranes of all cells are dynamic structures, the biomolecule composition of which is constantly changing as cells respond to environmental stimuli, alter protein and lipid composition, release waste products, take in nutrients, and perform many other cellular processes (Vereb et al., 2003; Watson, 2015). Throughout cellular life cycles, fragments of the outermost membrane are often shed as nanosized particles. In bacteria, these structures are often referred to as membrane vesicles (MVs) or outer membrane vesicles (OMVs), which will be referred to as MVs throughout for simplicity (a representative example of MVs from Lactobacillus casei is shown in Figure 1). As interest in these biological nanoparticles has grown in recent years, researchers have shown that MVs have broad biological activities, including host-microbe interactions, gene transfer, and community regulation (Kulp and Kuehn, 2010; Caruana and Walper, 2020).
Bacterial MVs have shown significant promise for applications such as vaccine development and potential therapeutic applications. Naturally occurring OMVs from Gram-negative bacteria have shown significant promise in the development of vaccines for bacteria such as Neisseria meningitidis and Burkholderia pseudomallei, pathogens that have proven challenging to vaccine and therapeutic development alike (Holst et al., 2009; Nieves et al., 2014). Recently, research groups have also shown that the MVs from some commensal bacteria can also modulate responses from host immune systems and stimulate host neurological systems (Mata Forsberg et al., 2019; Molina-Tijeras et al., 2019; Rodovalho et al., 2020). With growing capabilities in synthetic biology, the potential uses of MVs are steadily increasing as researchers have developed engineering strategies that allow for modification of genetic and cellular pathways to control the composition of nascent MVs. These efforts have led to new biomaterials for therapeutic applications, environmental decontamination, and other purposes (Alves et al., 2018; Qing et al., 2019).
The classification of lactic acid bacteria (LAB) encompasses several genera of bacteria with similar characteristics of acid-tolerance and fermentation capabilities. Many LAB are classified as generally regarded as safe (GRAS) and have been used in the production of food products for centuries. Additionally, several LAB have been recognized as beneficial to their host and are studied for their health benefits as probiotics leading to a large commercial market for probiotic supplements and foods. While live-bacterial cultures are the most commonly used form of probiotics, the potential for engineering or enriching for specific cellular components has led researchers to explore the use of purified MVs for controlled therapeutic applications (Seo et al., 2018; Molina-Tijeras et al., 2019; Dean et al., 2020).
There are numerous protocols for the purification of MVs from eukaryotic cells and Gram-negative bacteria, which have been the primary focus of MV research (Klimentova and Stulik 2015; Alves et al., 2017; Dauros Singorenko et al., 2017). Recently, there has been growing interest in the MVs of gut bacteria and the roles they may play in host and community interactions. LAB are Gram-positive bacteria and therefore have a significantly different membrane and peptidoglycan structure as compared to Gram-negative bacteria. While this may not specifically contribute to biophysical differences between Gram-negative and Gram-positive MVs, it has been shown that the MVs of some LAB species have a bimodal size distribution with an abundant population of smaller MVs in the 10-50 nm size range (Dean et al., 2019). Here, we describe protocols for the isolation and characterization of MVs that have proven successful for numerous LAB species. MVs are isolated from overnight LAB cultures via ultracentrifugation and then analyzed for concentration and relative size distribution using a NanoSight nanoparticle tracking instrument. Dynamic light scattering (DLS) is used as another way to measure size distribution and also to measure zeta potential (surface charge). Then, the protein content of MVs can be examined both qualitatively using SDS-PAGE and specifically by using mass spectrometry for proteomic analysis (schematic shown in Figure 2). While this manuscript describes work we have employed for LAB, these protocols could also be used for the isolation of MVs from a variety of microbial species.
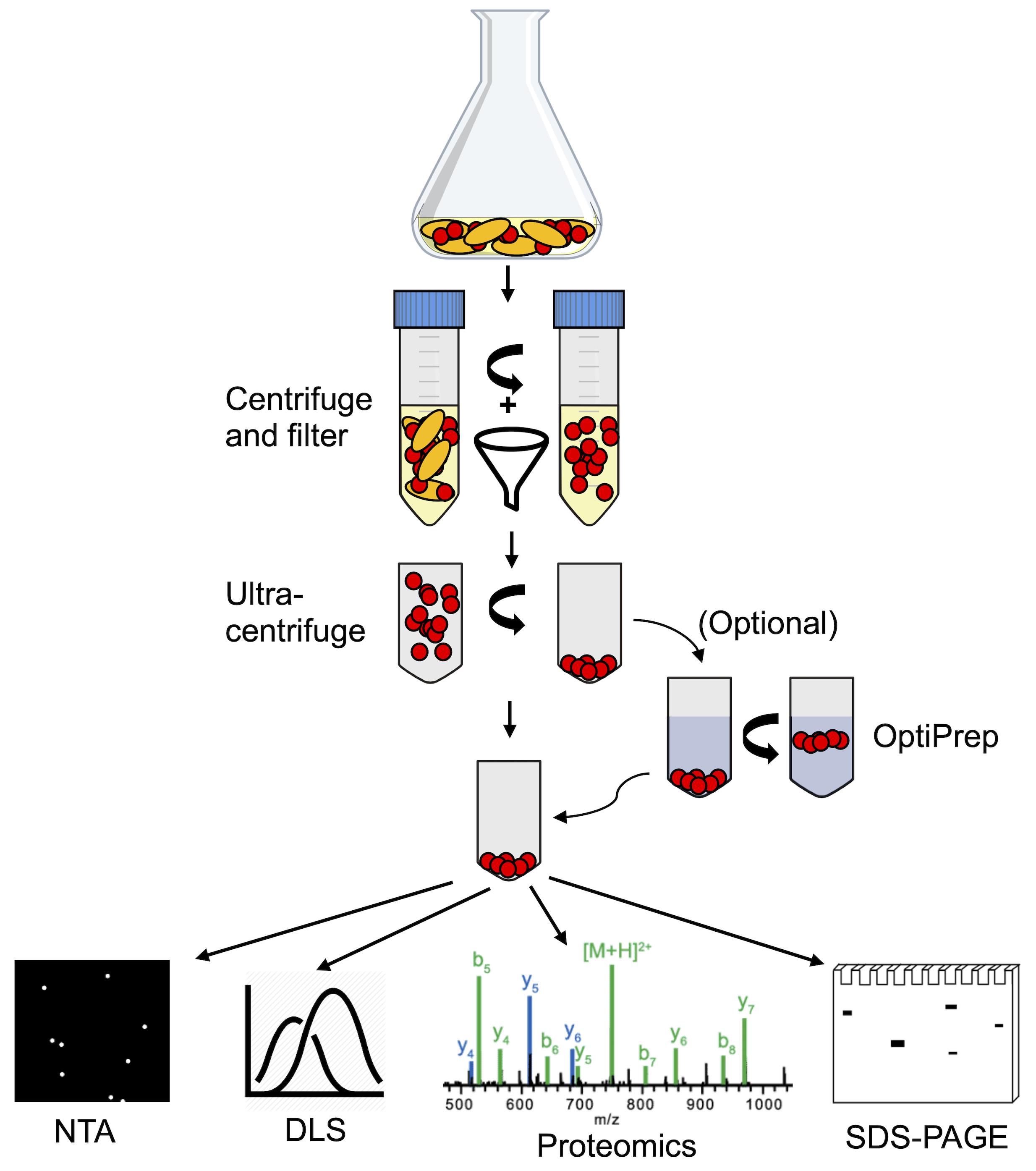
Figure 2. Schematic overview of the membrane vesicle isolation and characterization process. MVs are purified from Lactobacillus cultures initially via centrifugation and filtration, removing cells and large cellular debris. From the filtered supernatant, MVs are isolated by ultracentrifugation. Gradient centrifugation using OptiPrep medium can be applied to isolated MVs as an additional purification step. Purified MVs can then be characterized by nanoparticle tracking analysis (NTA), dynamic light scattering (DLS), SDS-PAGE protein gels, shotgun proteomics, or other methods.
Materials and Reagents
AnaeroJarTM 2.5 L jars (Oxoid®, ThermoFisher Scientific, catalog number: AG0025A)
AnaeroGenTM anaerobic gas generation sachets (Oxoid®, Thermo Fisher Scientific, catalog number: AN0025A)
Resazurin anaerobic indicator strips (Oxoid®, Thermo Fisher Scientific, catalog number: BR0055B)
100 mm sterile Petri dishes (Fisherbrand, Thermo Fisher Scientific, catalog number FB0875712)
Pyrex® Erlenmyer flasks 250 ml (CorningTM, Thermo Fisher Scientific, catalog number: CLS4980250)
50 ml conical centrifuge tubes (CorningTM Falcon, Thermo Fisher Scientific, catalog number: 14-432-22)
0.45 µm syringe filters (J. G. Finneran, catalog number: FEC0425PC)
30 ml sterile syringes (BD Slip tip sterile syringe; Thermo Fisher Scientific, catalog number: BD 302833)
38.5 ml ultracentrifuge tubes (Thinwall Ultra-Clear tubes; Beckman Coulter, catalog number: 344058)
5 ml ultracentrifuge tubes (Thinwall Ultra-Clear tubes; Beckman Coulter, catalog number: 344057)
1 ml needle-less syringes (Henke Sass Wolf, catalog number: 4010.200V0)
1.5 ml centrifuge tubes (Thermo Fisher Scientific, catalog number: P190411)
Disposable cells compatible with ZetaSizer Nanoseries (Malvern Panalytical, catalog number: DTS 1070)
Lactobacillus species, i.e.,
Lactobacillus acidophilus (ATCC 53544)
Lactobacillus casei (ATCC 393)
Lactobacillus reuteri (ATCC 23272)
Lactobacillus plantarum (ATCC BAA-793)
de Man, Rogosa, and Sharpe (MRS) media (Sigma-Aldrich, catalog number: 69966-500G, prepare according to the manufacturer’s instructions)
Tween® 80 (Sigma Aldrich, catalog number: P8074)
4-15% Mini-PROTEAN® TGX Pre-cast protein gels (Bio-Rad, catalog number:4561085)
GelCodeTM Blue Stain Reagent (Thermo Fisher Scientific, catalog number: PI24590)
10× phosphate-buffered saline (Thermo Fisher Scientific, catalog number: AM9625)
4× Laemmli sample buffer (Bio-Rad, catalog number: 1610747)
2-mercaptoethanol (Bio-Rad, catalog number: 161-0710)
SDS-PAGE running buffer (Bio-Rad, catalog number: 161-0772)
Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, catalog number: 23225)
1-propanol (Sigma-Aldrich, catalog number: 402893)
Ammonium bicarbonate (Sigma-Aldrich, catalog number: A6141)
Trypsin, sequencing grade (Promega, Fisher Scientific, catalog number: PRV5111)
Formic acid (Sigma-Aldrich, catalog number: 27001)
Acetonitrile (Sigma-Aldrich, catalog number: 34851)
OptiPrep medium (Progen Biotechnik GmbH, catalog number: 1114542)
SDS-PAGE Running Buffer (10× stock; use at 1×) (see Recipes)
Equipment
Incubator capable of maintaining 37°C (for example: Fisherbrand, Isotemp Microbiological Incubator)
Centrifuge with capacity for 50 ml conical tubes (for example: Beckman Coulter, Avanti JXN-30 using a JA-14.50 rotor)
Ultracentrifuge with capacity for 38.5 ml tubes, capable of 129,000 × g (for example: Sorvall WX Ultra 90 centrifuge using AH-629 rotor)
NanoSight LM10 (Malvern Panalytical, Worcestershire, UK)
ZetaSizer NanoSeries equipped with a HeNe laser source (λ = 633 nm) and avalanche photodiode (Malvern Panalytical, Worcestershire, UK)
Mini-PROTEIN® Tetra Vertical Electrophoresis Cell (Bio-Rad, catalog number: 1658004)
Speed-vac (for example: Thermo Fisher Scientific SC210A SpeedVac Concentrator, catalog number: SC210A-230)
Barocycler (Pressure Biosciences Inc., HUB 440-SW16, Easton, MA, US)
Orbitrap LC-MS/MS system (for example: Thermo Scientific Orbitrap Fusion Lumos equipped with a Nanospray Flex Ion Source (Thermo Fisher Scientific, catalog number: ES071)
Autosampler (for example: Thermo Scientific Dionex UltiMate 3000 Rapid Separation Well Plate Autosampler (Thermo Fisher Scientific, catalog number: 5840.0010)
Ultra-high performance liquid chromatography (UHPLC) system (for example: Dionex Ultimate 3000 RSLCnano system, Thermo Fisher Scientific, catalog number: ULTIM3000RSLCNANO)
Software
NTA 2.3 Nanoparticle Tracking and Analysis software (Malvern Panalytical, Worcestershire, UK)
Dispersion Technology Software (DTS, Malvern Panalytical, Worcestershire, UK) used for dynamic light scattering analysis
Scaffold version 4.8.2 (Proteome Science Inc., Portland, Oregon, US), used for protein identification after mass spectrometry
Mascot (version 2.6.1, Matrix Science, London, UK), used for protein identification after mass spectrometry
X! Tandem (version 1.7.18) used for protein identification after mass spectrometry
R (https://cran.r-project.org/) and relevant R packages: Peptides, Limma, and ggplot2. Used for biochemical analysis of identified proteins, statistical analysis, and generation of volcano plots or other visualizations of the data
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Caruana, J. C., Dean, S. N. and Walper, S. A. (2021). Isolation and Characterization of Membrane Vesicles from Lactobacillus Species. Bio-protocol 11(17): e4145. DOI: 10.21769/BioProtoc.4145.
分类
微生物学 > 微生物细胞生物学 > 细胞器分离
细胞生物学 > 细胞器分离 > 胞外囊泡
生物科学 > 生物技术 > 微生物技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













