- EN - English
- CN - 中文
Neutral Comet Assay to Detect and Quantitate DNA Double-Strand Breaks in Hematopoietic Stem Cells
检测和定量造血干细胞DNA双链断裂的中性彗星试验
(*contributed equally to this work) 发布: 2021年08月20日第11卷第16期 DOI: 10.21769/BioProtoc.4130 浏览次数: 4917
评审: Giusy TornilloRakesh BamAnonymous reviewer(s)
Abstract
In vertebrates, hematopoietic stem cells (HSCs) regulate the supply of blood cells throughout the lifetime and help to maintain homeostasis. Due to their long lifespan, genetic integrity is paramount for these cells, and accordingly, a number of stem cell-specific mechanisms are employed. However, HSCs tend to show more DNA damage with increasing age due to an imbalance between proliferation rates and DNA damage responses. The comet assay is the most common and reliable method to study DNA strand breaks at the single-cell level. This procedure is based on the electrophoresis of agarose-embedded lysed cells. Following the electrophoretic mobilization of DNA, it is stained with fluorescent DNA-binding dye. Broken DNA strands migrate based on fragment size and form a tail-like structure called “the comet,” whereas intact nuclear DNA remains a part of the head of the comet. Since the alkaline comet assay fails to differentiate between single and double-strand breaks (DSBs), we used a neutral comet assay to quantitate the DSBs in HSCs upon aging and other physiological stresses. The protocol presented here provides procedural details on this highly sensitive, rapid, and cost-effective assay, which can be used for rare populations of cells such as HSCs.
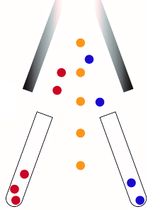
Graphical abstract:

The neutral comet assay is an extremely useful tool that allows the detection and quantitation of double-strand DNA breaks at the single-cell level. The graphical abstract represents a flowchart for the neutral comet assay procedure.
Background
During every round of cell proliferation, the genomic content is faithfully replicated, albeit with some errors. These errors lead to changes or modifications in the structure and chemical nature of genomic DNA. The replication machinery is tightly associated with DNA damage repair (DDR) pathways, thereby recovering cells from such damage (Chatterjee and Walker, 2017). Alternatively, the DNA damage response pathways can induce apoptosis (Nowsheen and Yang, 2012); however, some DNA damage is undetected and unrepaired, accumulating over a period of time. Accumulation of this DNA damage during aging poses a serious challenge to the healthy functioning of a variety of tissues, including hematopoietic systems. Recent studies have indicated that accumulation of age-related physiological changes in the stem cell population leads to alterations in immune system function (Oh et al., 2014). In addition, several age-associated malignancies have been linked to DNA damage accumulation in the stem cell compartment (Beerman, 2017). Hematopoietic stem cells have cytoprotective and genoprotective mechanisms to safeguard their long-lasting functional potential; however, it has been demonstrated that DNA damage accumulates in HSCs during the aging process. Several research groups have proposed that this accumulation is due to increased proliferation rates in old HSCs. In contrast, others show that long-term dormancy may prevent rare DNA damage from accumulating due to lack of replication-associated repair (Beerman et al., 2014).
To understand the fundamental processes involved in DNA damage accumulation and repair, quantitation of DNA damage is vital (Lu et al., 2017). In 1984, Ostling and Johanson demonstrated the electrophoretic mobility of DNA fragments from the core nuclei (Ostling and Johanson, 1984). When single cells embedded in low-melting-point agarose are electrophoresed, the DNA-damaged fragments move faster, giving rise to tail-like structures resembling a comet. The intact part of the DNA is called the head of the comet, and the trail produced due to impaired DNA is known as the tail of the comet. This single-cell gel electrophoresis assay, also known as the comet assay, is one of the most reliable and commonly used methods for analyzing DNA damage in cells. It has been successfully applied to understand the processes involved in genotoxicity, molecular epidemiology, and human bio-monitoring, along with solving basic biology questions regarding DNA damage and repair (Collins, 2004). This technique was further modified by Singh et al., who showed a substantial increase in reproducibility under alkaline conditions (Lu et al., 2017). At alkaline pH, the sensitive sites are knocked down and the strands break due to distortion in their conformation. Therefore, the alkaline comet assay is commonly used to analyze strand breaks, DNA-protein crosslinks, and alkali-labile sites; nevertheless, the specificity for double-strand breaks is compromised (Lu et al., 2017). To overcome this issue, the neutral comet assay was further developed (Calini et al., 2002); it protects the DNA double strands from distortion, thus aiding in the specific detection of double-strand DNA damage. These DNA strand breaks result in the lengthening of DNA loops in genomic DNA, which form a comet-like tail in an electrophoretic mobility assay (Cortes-Gutierrez et al., 2012). Because double-strand DNA breaks lead to mutations and chromosomal abnormalities, it is important to detect and quantitate them. The neutral comet assay is of immense importance and is highly efficient for in vivo and ex vivo systems (Sharma et al., 2011). The comets formed due to electrophoretic mobility can be visualized by fluorescence microscopy. The percentage of DNA damage can be estimated from the percentage of DNA present in the comet tail since they are directly proportional to one other. With advancements in technology such as fluorescent staining and automated comet scoring software, the comet assay has become popular for detecting DNA damage (Beedanagari, 2017). In the case of rare cell populations such as HSCs, the neutral comet assay has emerged as a promising technique to study DNA damage responses due to physiological, pathological, and age-associated stresses (Hazlehurst and Dalton, 2001; Milyavsky et al., 2010). The present protocol describes the method to assess DNA damage in freshly sorted hematopoietic stem cells from mice bone marrow.
Materials and Reagents
Autoclaved lint-free tissue paper
60-mm tissue culture plates (Eppendorf, catalog number: 0030701119)
26 G needle (BD PrecisionGlide, BD Medical (S) Pvt Ltd, catalog number: 302806)
1-ml syringe (BD Medical (S) Pvt Ltd, catalog number: 303060)
40-µm nylon strainer (Falcon, catalog number: 352340)
Bone marrow cells from mice
FACS tubes (Falcon, catalog number: 352054)
Comet slides (CometSlidesTM 20 well, Trevigen, catalog number: 4252-02K-01)
MACS apparatus
MS column (MACS, Miltenyi Biotec, catalog number: 130-042-201)
MACS multistand (MACS, Miltenyi Biotec, catalog number: 002139)
MiniMACSTM separator (MACS, Miltenyi Biotec, catalog number: 130-042-102)
Antibodies
APC-conjugated lineage antibody cocktail [2:100 concentration] (BD PharmingenTM, BD Biosciences, catalog number: 51-9003632)
Anti-mouse c-kit PE [1:100 concentration] (Biolegend, catalog number: 105807)
Anti-mouse Sca-1 PerCP-Cy5.5 [1:100 concentration] (Biolegend, catalog number: 122524)
1% agarose (Sigma-Aldrich, catalog number: A9539)
0.75% low-melting-point agarose (LM agarose) (Sigma-Aldrich, catalog number: A9414)
70% ethanol (Changshu Hongsheng Fine Chemical Co., Ltd, catalog number: 1170)
SYBR® Gold staining solution (ThermoFisher Scientific, Invitrogen, catalog number: S33102)
10× RBC lysis buffer (Biolegend, catalog number: 420301)
Lysis buffer (see Recipes)
1× neutral electrophoresis buffer (see Recipes)
DNA precipitation solution (see Recipes)
1× phosphate-buffered saline (PBS) (see Recipes)
Equipment
Electrophoresis unit (Mini-Sub® Cell GT, Bio-Rad, catalog number: 329BR014542)
Epifluorescence microscope (Olympus IX2-ILL100, OLYMPUS CORPORATION, catalog number: 0M04213)
Brightfield microscope (Primovert, Ziess, catalog number: 3842010199)
Software
ImageJ – Open Comet (Benjamin M. Gyori and Gireedhar Venkatachalam, www.cometbio.org)
Excel 2016 (Microsoft Corporation)
GraphPad Prism (GraphPad Software, Inc., www.graphpad.com)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Roy, I. M., Nadar, P. S. and Khurana, S. (2021). Neutral Comet Assay to Detect and Quantitate DNA Double-Strand Breaks in Hematopoietic Stem Cells. Bio-protocol 11(16): e4130. DOI: 10.21769/BioProtoc.4130.
分类
干细胞 > 成体干细胞 > 造血干细胞
癌症生物学 > 基因组不稳定性及突变
分子生物学 > DNA > DNA 损伤和修复
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link