- EN - English
- CN - 中文
Unraveling the Physicochemical Determinants of Protein Liquid-liquid Phase Separation by Nanoscale Infrared Vibrational Spectroscopy
利用纳米尺度红外振动光谱揭示蛋白质液-液相分离的理化决定因素
发布: 2021年08月20日第11卷第16期 DOI: 10.21769/BioProtoc.4122 浏览次数: 7844
评审: Steven BoeynaemsWillem VanderlindenAnonymous reviewer(s)
Abstract
The phenomenon of reversible liquid-liquid phase separation of proteins underlies the formation of membraneless organelles, which are crucial for cellular processes such as signalling and transport. In addition, it is also of great interest to uncover the mechanisms of further irreversible maturation of the functional dense liquid phase into aberrant insoluble assemblies due to its implication in human disease. Recent advances in methods based on atomic force microscopy (AFM) have made it possible to study protein condensates at the nanometer level, providing unprecedented information on the nature of the intermolecular interactions governing phase separation. Here, we provide an in-depth description of a protocol for the characterisation of the morphology, stiffness, and chemical properties of protein condensates using infrared nanospectroscopy (AFM-IR).
Keywords: Liquid-liquid phase separation (液-液分离阶段)Background
Liquid-liquid phase separation (LLPS, Figure 1) is a fundamental process resulting in the formation of membraneless subcellular bodies, such as nucleoli and germ granules (Hyman et al., 2014; Banani et al., 2017). This reversible transition, during which proteins condense into a dense liquid phase or droplet within the cell, is involved in many aspects of cellular organisation and function, such as mRNA processing and signalling (Murakami et al., 2015). It has been realised that most of the proteins that undergo LLPS contain intrinsically disordered domains (IDRs), which are involved in networks of multivalent interactions that underpin the condensation process (Li et al., 2012). However, dysregulation of this process can lead to the formation of solid hydrogel-like inclusions, which are implicated in human disease. Such is the case of fused in sarcoma (FUS), an RNA-binding protein implicated in the onset of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) (Patel et al., 2015).
The LLPS behaviour of protein condensates has typically been studied using optical and fluorescence microscopy methods, as well as bulk characterisation of properties in solution, such as turbidity measurements (Alberti et al., 2019). These approaches have shown the effect of external factors on the modulation of phase separation, revealing the influence of protein concentration, pH, salt type and concentration, and small molecules (Hyman et al., 2014; Murakami et al., 2015; Patel et al., 2017; Ruggeri et al., 2018; Alberti et al., 2019). A further understanding of the process of LLPS, in particular the fundamental molecular interactions determining protein condensation and the inner structure of the individual protein condensates, is hampered by their intrinsic transient nature and sub-micrometer scale dimensions. However, conventional approaches, which are limited by their spatial resolution and the need for highly concentrated samples, cannot probe molecular mechanisms and internal factors determining LLPS at the individual condensate and sub-condensate levels.

Figure 1. Schematic of protein aggregation vs. liquid-liquid phase separation and liquid-to-solid transition
In our previous and current work (Müller et al., 2014; Ruggeri et al., 2015, 2016a, 2018, 2020a, and 2020b; Ramer et al., 2018; Lipiec et al., 2019), we have demonstrated that infrared nanospectroscopy (AFM-IR) can provide new and complementary information, as compared with bulk methods, regarding the phenomenon of protein LLPS at the sub-condensate nanoscale level. This information can then be successfully exploited to address fundamental knowledge gaps in the physicochemical determinants of this process (Müller et al., 2014; Ruggeri et al., 2015, 2016a, 2016b, 2018, 2020a; Dazzi and Prater, 2017; Lipiec et al., 2019). AFM-IR combines the capabilities of infrared spectroscopy (IR) and atomic force microscopy (AFM) to allow for the simultaneous characterisation of morphology, stiffness, and chemical properties of individual droplets, and even of different regions within the same droplet, thereby providing mechanistic insight into their inner biophysical properties and formation.
The AFM-IR system bases its function on scanning probe microscopy (Figure 2). Briefly, during morphology acquisition, the tip and cantilever in contact with the sample can be used to acquire nanomechanical and nanospectroscopy information. The relative stiffness of the sample is evaluated by considering the tip and the sample in contact as a system of coupled springs. The contact resonance frequency of these springs is monotonically related to the intrinsic stiffness of the sample (Volpatti et al., 2016; Qamar et al., 2018). A stiffer sample would cause an increase in contact resonance, which we can term ‘frequency shift’; thus, the mechanical properties at the sub-droplet nanoscale level can be measured (Qamar et al., 2018; Shen et al., 2020). To retrieve chemical information in the form of nanoscale-resolved maps and localised spectra, the tip is used as a detector of photothermal expansion. If a sample absorbs IR light at a specific wavelength, the chemical bonds absorbing the light will vibrate; this vibration is dissipated as thermal heat, producing a sample expansion that can be measured by the AFM tip in contact with the sample. The response of the cantilever is proportional to the IR absorption (Ruggeri et al., 2016b). Localised IR spectra can be acquired by holding the tip at a fixed position on the sample while sweeping the frequency of the IR light. Similarly, IR maps of whole droplets can also be acquired by measuring the thermal expansion at a fixed wavelength during the measurement of sample morphology. AFM-IR is capable of acquiring chemical information down to the single protein molecule scale (Ruggeri et al., 2020a). In the case of LLPS of proteins, it is relevant to investigate the vibrations associated with the amide band I, which is related to C=O stretching (80%) and is therefore sensitive to the conformation and hydrogen bonding of the protein backbone. Thus, the shape of the amide band I is studied to reveal secondary and quaternary structural information (Ruggeri et al., 2015; 2016a and 2020a).
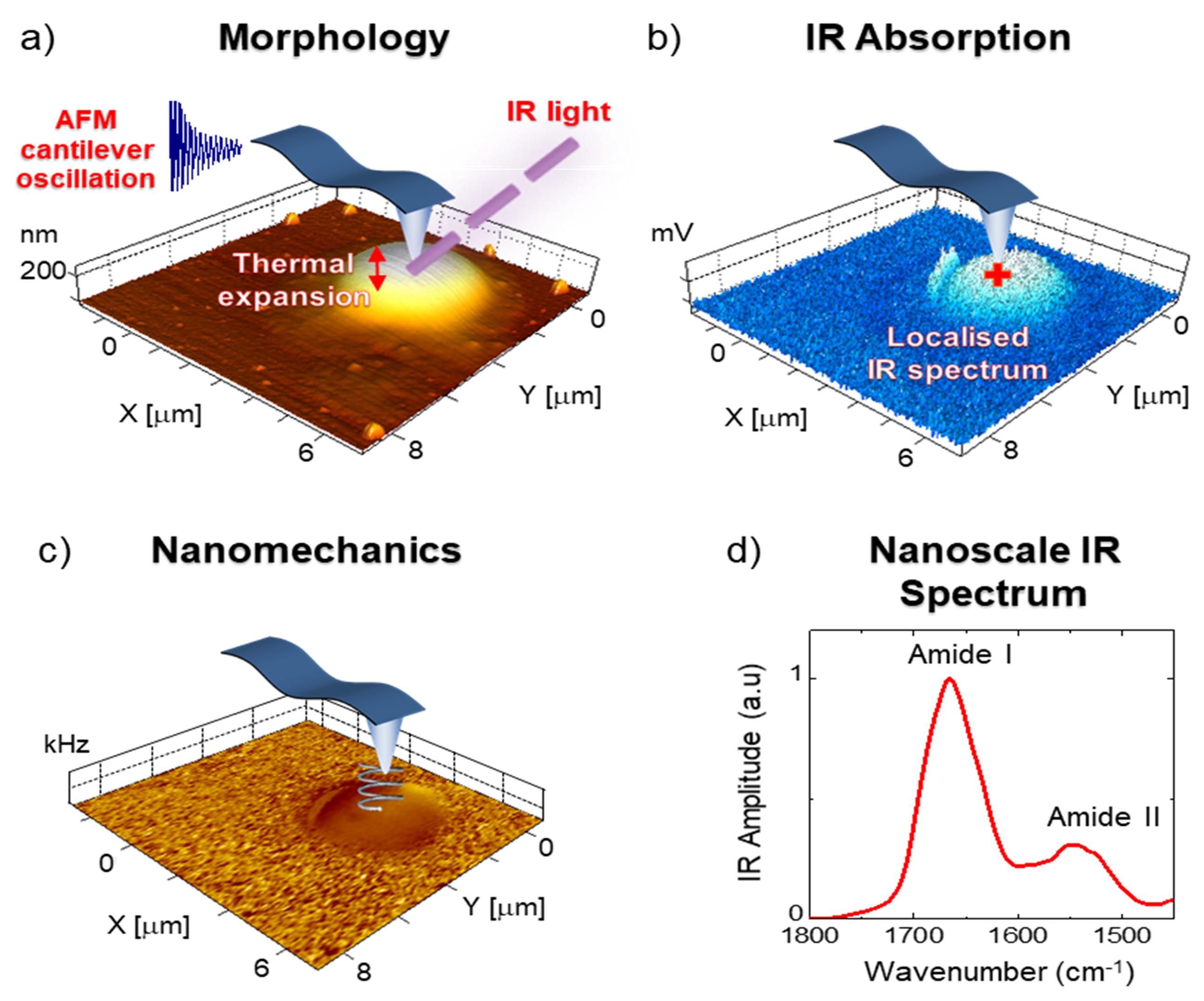
Figure 2. AFM-IR nanoscale chemical imaging and spectroscopy principles for a liquid-liquid phase-separated droplet. Absorbed IR light causes thermal expansion of the sample exciting the AFM cantilever, whose thermomechanical response is proportional to the absorbed light. Scanning the cantilever on the sample while fixing the laser wavelength enables one to simultaneously obtain nanoscale-resolved a) morphology, b) chemical (IR absorption), and c) mechanical maps. d) nanoscale-localised (red cross) IR spectra are then obtained by sweeping the laser wavelength while fixing the position of the cantilever.
AFM-IR has recently been successfully exploited to study protein droplets for drug delivery purposes, to evaluate the effect of post-translational modifications, specifically methylation, and to investigate the interactions and phase-separation behaviour, in addition to the effect of shear, on liquid phase-separated condensates (Müller et al., 2014; Volpatti et al., 2016). In an initial study involving the application of AFM-IR to phase-separated droplets, it was shown that a change in methylation state alters the structural and biophysical state of condensates of FUS, the dysfunction of which impairs neuronal function. The study quantitatively compared the structural state of the methylated and hypomethylated condensates, showing an increased content of antiparallel β-sheet in the latter. Hypomethylated FUS also showed heterogeneity with the coexistence of liquid and gel-like states within a single condensate, suggesting a possible interconversion between the two states (Qamar et al., 2018). A more recent study reported a liquid-to-solid transition through the application of shear, which led to the formation of stable fibres that were more anistropically ordered and had a higher β-sheet content and intermolecular hydrogen bonding compared with spherical liquid-liquid phase-separated condensates (Shen et al., 2020). In this study, infrared nanopolarimetry was applied to study the internal structural organisation of the shear-formed FUS fibrils.
Here, we describe the protocol followed for the controlled, in-depth characterisation of the morphology, stiffness, and chemical properties of phase-separated condensates using AFM-IR.
Materials and Reagents
FUS sample at a concentration of 1-10 μM (the production and purification of FUS is discussed in previously published protocols (Qamar et al., 2018)
ZnSe window (Thorlabs, catalog number: WG70530)
MilliQ water
Equipment
AFM-IR with thermomechanical detection: nanoIR1, nanoIR2, or nanoIR3 (Anasys Instruments, Bruker, USA)
PR-EX-nIR2 AFM probes: silicon, gold-coated, with a nominal radius of 30 nm and a spring constant of 0.2 N/m (for contact mode measurements; Anasys, Bruker, USA)
P10 Pipette (Starlabs, UK)
Software
In-built nanoIR software (Bruker, USA)
SPIP (Image Metrology, Denmark, https://www.imagemet.com/products/spip/)
OriginPRO (Origin Labs, USA, https://www.originlab.com/origin)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Ruggeri, F. S., Miller, A. M., Vendruscolo, M. and Knowles, T. P. J. (2021). Unraveling the Physicochemical Determinants of Protein Liquid-liquid Phase Separation by Nanoscale Infrared Vibrational Spectroscopy. Bio-protocol 11(16): e4122. DOI: 10.21769/BioProtoc.4122.
分类
生物物理学 > 显微技术 > 原子力显微镜
神经科学 > 神经系统疾病 > 神经退行性病变
分子生物学 > 蛋白质 > 蛋白质-蛋白质相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link