- EN - English
- CN - 中文
Monitoring Protein Splicing Using In-gel Fluorescence Immediately Following SDS-PAGE
SDS-PAGE后立即使用凝胶内荧光监测蛋白质剪接
发布: 2021年08月20日第11卷第16期 DOI: 10.21769/BioProtoc.4121 浏览次数: 4277
评审: Jan-Ulrik DahlFernando A Gonzales-ZubiateAnonymous reviewer(s)
Abstract
Inteins garner significant interest from both basic and applied researchers due to their unique catalytic abilities. Herein, we describe a protocol for accurately monitoring protein splicing without purification using in-gel fluorescence immediately following Tris-Glycine SDS-PAGE. Following expression in Escherichia coli, cells are lysed by sonication, cell supernatants are separated using Tris-Glycine SDS-PAGE, and superfolder GFP (sfGFP) fluorescence is directly visualized within gels. This method is rapid, with sfGFP immediately imaged following SDS-PAGE without staining. Further, sfGFP can be specifically detected in complex samples such as E. coli cell supernatants, proteins run at expected masses, and all steps can be performed at ambient temperature. This strategy is broadly applicable beyond the study of protein splicing and can be used for sensitive and specific visualization of superfolder sfGFP-tagged proteins in-gel.
Keywords: In-gel fluorescence (凝固态荧光)Background
Phylogenetically widespread in the microbial world, inteins (intervening proteins) have generated substantial interest as agents of autocatalysis, drivers of evolution, antibacterial and antifungal targets, and for biotechnological application (Lennon and Belfort, 2017). In the protein splicing reaction, the intein is removed by rearranging two peptide bonds and connecting the surrounding sequences, known as N-and C-exteins, with a peptide bond. While this process can occur in the absence of any external factors, its rate can be highly variable depending on conditions. Recently, compelling evidence has demonstrated that inteins can act as environmental sensors that regulate protein function in response to conditions such as metals, heat, salt, oxidative stress, and DNA damage (Belfort, 2017). Thus, significant interest from both basic and applied communities necessitate the investigation of inteins.
The study of intein catalysis is most often achieved using protein splicing reporters. The only strict requirement of these reporters is the presence of a cysteine, serine, or threonine as the first residue immediately following the intein (Mills et al., 2014). We have utilized a reporter, referred to as MIG (MBP-Intein-GFP), to study the activity of a variety of inteins (Topilina et al., 2015a and 2015b; Kelley et al., 2016 and 2018; Lennon et al., 2018; Green et al., 2019; Woods et al., 2020). In the MIG reporter, the intein (surrounded by 10 native extein residues) is flanked by the Maltose Binding Protein (MBP) as the N-extein and superfolder GFP (sfGFP) as the C-extein (Figure 1). Products with sfGFP attached are detected using in-gel fluorescence immediately following SDS-PAGE. sfGFP-tagged products (those containing a C-extein) allow for the monitoring of all possible intein reactions, including splicing (resulting in fluorescent ligated exteins), N-terminal cleavage (resulting in fluorescent intein-C-extein), and C-terminal cleavage (resulting in fluorescent C-extein) (Figure 1). Depending on the intein and reaction conditions, not all products may be detected. The major advantages of our sfGFP-based reporter, compared to other protein splicing reporters, is that there is no need for purification following expression and products can be visualized without staining.

Figure 1. Maltose binding protein-Intein-sfGFP (MIG) protein splicing reporter. Products of splicing, N-terminal cleavage, and C-terminal cleavage that are visible (i.e., sfGFP-containing) using in-gel fluorescence are shown for each reaction.
Moreover, the use of in-gel fluorescence is broadly applicable beyond the study of protein splicing. This in-gel fluorescence protocol can be used to accurately examine the size of any sfGFP-tagged protein expressed in E. coli without purification. For example, this strategy could be used to study the localization of proteins in different cellular fractions, such as the cytoplasm, periplasm, and membrane of E. coli. Similar procedures have been described for visualization of GFP-tagged proteins. The protocol described herein differs as it allows visualization without staining (Pristov et al., 2015) and does not require running gels at 4°C (Bird et al., 2015). Several critical parameters relating to sfGFP-detection are also addressed. As GFP structure is required to maintain its fluorescent signal, it is critical that samples are not heated prior to SDS-PAGE (Figure 2). Further, we find that the use of Tris-Glycine rather than Bis-Tris gels improves sensitivity (Figure 2).
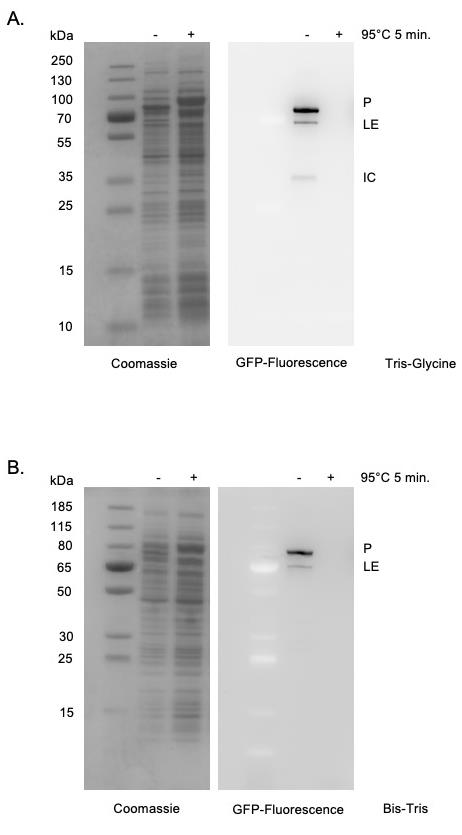
Figure 2. sfGFP detection within SDS-PAGE. Tris-Glycine gels (Panel A) are more sensitive than Bis-Tris gels (Panel B) for detecting in-gel sfGFP signals as indicated by stronger signal, lower background, as well as detection of the minor product (IC) only in Tris-Glycine gels. sfGFP fluorescence is specifically detected in cell supernatants without purification. Heating samples at 95°C for 5 min results in a loss of fluorescent signal. Left, Coomassie stained. Right, sfGFP signal. Expected molecular weights of products are as follows: Precursor (P) 88.5 kDa; Ligated exteins (LE) 74.0 kDa; Intein-C-extein (IC) 42.6 kDa.
Materials and Reagents
10, 20, and 1,000 μl Pipette tips (USA Scientific, Inc. TipOne, catalog numbers: 1111-3800,1120-1810, and 1111-2820)
1.5 ml tubes (Eppendorf, catalog number: 022363204)
50 ml conical tubes (Corning, catalog number: 352070)
Gel loading tips (USA Scientific, catalog number: 1022-0000)
Culture tube (Fisher Scientific, catalog number: 14-961-31)
10 ml syringe (BD, catalog number: 309604)
0.22 μm syringe filter (Fisher Scientific, catalog number: 09-720-3)
Mini-PROTEAN TGX Precast Protein Gels, Tris-Glycine, 8-16% (Bio-Rad Laboratories, catalog number: 4561106)
4× Laemmli Sample Buffer (Bio-Rad Laboratories, catalog number: 1610747)
10× Tris/Glycine/SDS electrophoresis buffer (Bio-Rad Laboratories, catalog number: 1610732)
MIG (or sfGFP) expression strain (available upon request)
Tris, 1.0 M buffer solution, pH 7.5, 0.2-μm filtered (Alfa Aesar, catalog number: J62993)
Tris, 1.0 M buffer solution, pH 8.5, 0.2-μm filtered (Alfa Aesar, catalog number: J61038)
Glycerol (Fisher Scientific, catalog number: BP229)
Zinc acetate dihydrate (Fisher Scientific, catalog number: S25634)
LB broth base (Difco, catalog number: 244610)
IPTG (Goldbio, catalog number: 12481C25)
Chloramphenicol (Sigma, catalog number: C0378)
MIG Buffer (see Recipes)
1× Tris/Glycine/SDS Running Buffer (see Recipes)
1 M Isopropyl β-D-1-thiogalactopyranoside (IPTG) (see Recipes)
25 mg/ml Chloramphenicol stock solution (i.e., 1,000×, see Recipes)
LB medium (see Recipes)
10 mM Zinc acetate solution (see Recipes)
Equipment
Cell Sonicator (Heat Systems, model: XL-2020)
FB300 Power Supply (Fisher Scientific, catalog number: S65533Q)
Mini-PROTEAN Tetra Vertical Electrophoresis Cell for Mini Precast Gels, 4-gel (Bio-Rad Laboratories, catalog number: 1658004)
Amersham Imager 600 (GE, catalog number: 29-0834-61)
P20 Pipetman (Gilson, catalog number: FA10003M)
P1000 Pipetman (Gilson, catalog number: FA10006M)
Microcentrifuge (Eppendorf, catalog number: 5420000113)
Centrifuge (Fisher Scientific, catalog number: 75005194)
Benchtop Refrigerated Shaking Incubator (Thermo Scientific, catalog number: SHKE4000)
Software
ImageJ (software free to download at https://imagej.nih.gov)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Weinberger II, J. and Lennon, C. W. (2021). Monitoring Protein Splicing Using In-gel Fluorescence Immediately Following SDS-PAGE. Bio-protocol 11(16): e4121. DOI: 10.21769/BioProtoc.4121.
分类
生物化学 > 蛋白质 > 标记
微生物学 > 微生物生物化学
分子生物学 > 蛋白质 > 活性
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link