- EN - English
- CN - 中文
A Simple Spatial-independent Associative and Reversal Learning Task in Mice
小鼠的一个简单的空间独立的联想和逆转学习任务
(*contributed equally to this work) 发布: 2021年08月05日第11卷第15期 DOI: 10.21769/BioProtoc.4108 浏览次数: 3680
评审: Arnau Busquets-GarciaZheng Zachory Wei

相关实验方案
基于 rAAV-α-Syn 与 α-Syn 预成纤维共同构建的帕金森病一体化小鼠模型
Santhosh Kumar Subramanya [...] Poonam Thakur
2025年12月05日 1584 阅读
Abstract
The ability to adapt one's behavior in response to changing circumstances, or cognitive flexibility, is often altered in neuropsychiatric and neurodevelopmental conditions. In rodents, cognitive flexibility is frequently assessed using associative learning paradigms with a reversal component. The majority of existing protocols rely on unrestrictive exploration with no discouragement of wrong responses and are often influenced by spatial cues, at least during the test's learning phase. Here, we present a rewarded contingency discrimination learning test that minimizes the task's spatial component and contains an element that actively discourages pure exploratory responses. The method described herein is a manual version that can be performed using home-made equipment, but the test setup is amenable to automatization and can be adapted to address more complex cognitive demands, including conditional associative learning, attentional set formation, and attention shifting.
Keywords: Associative learning (联想学习)Background
Contingency reversal learning tasks, where an animal is trained to associate a cue with a reward or a punishment that later is reversed, are frequently used to study neural mechanisms of cognitive flexibility (Izquierdo et al., 2017). In mice, discrimination and reversal learning can be challenging to assess, partly because of two salient and contrasting aspects of species-specific mouse behavior: novelty-induced anxiety (Crusio, 2001) and the tendency to follow incidentally learned spatial cues (Hebert et al., 2017). The natural defensive behavior of mice limits the use of tests based on negative reinforcement, as they reduce exploration and almost invariably result in the animal not performing any response at all. For example, the addition of punishments to incorrect responses appears to worsen reversal learning proficiency as assessed in a touchscreen paradigm (Jager et al., 2020). On top of this, there are ethical concerns about using negative reinforcements in animal experiments. The use of only positive reinforcers, such as food rewards, also has its drawbacks since the motivation to respond to a reward-associated stimulus may change over time along with satiety (Goltstein et al., 2018).
Furthermore, rodents have a natural tendency to alternate between responses (Lalonde, 2002; Deacon and Rawlins, 2006), and in particular, young individuals tend to explore the consequences of alternative non-rewarded responses provided that these do not trigger an openly aversive effect (Walker et al., 1955; Mechan et al., 2009; Sanderson and Bannerman, 2012). In most operant tasks assessing discrimination learning in mice, wrong responses are simply not rewarded and do not require a corrective action by the mouse, which has to “understand” that the given response was wrong by the absence of a consequence. Time-outs appear insufficient to discourage animals from attempting alternative responses, and these alternative attempts may confound the animals’ cognitive ability with its exploratory attitude (Luo et al., 2020). The introduction of an operant response to re-start a trial is a partial remedy to this drawback; however, the presence of a negative, but not aversive, cue associated with a wrong response is the best way to achieve the highest possible proficiencies in these cognitive tasks.
Reversal learning tasks designed for rodents are often conducted in mazes or operant conditioning chambers, where the rewarded (S+) and non-rewarded (S-) stimuli are set at a physical distance sufficient to be discriminated based on their position. When discrimination between the stimuli is not based on location (e.g., shape, odor, or texture discrimination), the incidental learning of their positions in space generates interference with spatial rules, at least during the test’s procedural learning steps. This phenomenon is known as overshadowing and occurs when a second salient cue, such as a spatial one, is in conflict with the designed S+ stimuli (Sánchez-Moreno et al., 1999). Indeed, it has long been established that rats follow spatial cues as a first strategy to retrieve rewards in a maze (Packard and McGaugh, 1996), and some individuals tend to follow spatial strategies even after having been trained in a non-spatial cue-response task in a water maze (Packard and McGaugh, 1992). Similarly, when initially trained to discriminate odors in the “classical” two-chamber setting of the attentional set-shifting task (Bissonette and Powell, 2012), rodents invariably start following spatial cues to figure out where to find a reward (Tait et al., 2018). Suppression of this spatial bias interfering with the conditioning stimulus may require additional days of training and may – paradoxically – result in worse performance of animals that usually rely more on hippocampal-dependent spatial information: an ecologically more relevant feature when seeking for a reward-related contingency in rodents (Devan and White, 1999; Chang and Gold, 2003). The interference of incidentally learned spatial information can be so relevant that it can result in paradoxically enhanced discrimination learning in hippocampal lesioned mice (Sanderson et al., 2012).
Here, we present a protocol to assess discrimination learning and contingency reversal in a simple home-made setup, under conditions that improve on both the previously described limitations of most current discrimination learning devices. The present protocol minimizes the interference of non-relevant spatial information by concentrating the S+ and S- stimuli on a single, small pedestal placed in a compartment of the arena that animals will recognize as a single “reward zone.” This is a significant difference compared to the arenas used for the classical attentional set-shifting tasks, where the S+ and S- are presented in two separate adjacent compartments (e.g., Colacicco et al., 2002). In addition, we apply a negative response signal by introducing a sliding lid moving inside the test box upon attempts to reach the non-rewarded vial. This step is equivalent to closing the reward receptacle, an operation that can be performed on some other operant tasks (e.g., Krackow et al., 2010). Furthermore, it also acts as a “trial-ended” signal and solicits a withdrawal response in the mouse to the waiting zone, thus prompting a new trial.
Materials and Reagents
15-ml Falcon tubes with lids (BD Biosciences, catalog number: 352096)
Plastic (1.5 mm) and Plexiglas (4 mm) panels (Jumbo-Markt AG, Switzerland)
Sandpaper (Jumbo-Markt AG, Switzerland)
Teflon tape (Jumbo-Markt AG, Switzerland)
Wood block (Jumbo-Markt AG, Switzerland)
Iron plate (0.5 mm) (Jumbo-Markt AG, Switzerland)
Neodymium disc magnets Ø 2 mm, height 2 mm (Jumbo-Markt AG, Switzerland)
Condensed milk cream (9% milk fat, 57% sugar, 8% milk proteins) (Migros, Zürich, Switzerland)
Felt-tip pen (Sharpie Fine, Atlanta, GA, USA)
Video camera (Ikegami ICD47E, 41460 Neuss, Germany)
Monitor (Samsung, model: 710 WP S)
Equipment
Test arena
The test takes place in an arena made with a plastic crate, such as a 20-L EURO Rako box (https://www.utzgroup.ch/stacking-container-rako-400-300-220-mm-161559/). The inner dimensions are 37 × 27 cm, 21.5 cm height. The box is divided into two halves by inserting a 0.4 × 27 cm Plexiglas wall, in which a 5 × 5 cm slot has been cut. One half is the waiting zone, and the other is the reward zone. The opening between the zones can be closed by an opaque polypropylene sliding guillotine door (Figure 1).

Figure 1. Top view of the arenaOn one of the box's short sides, two 52 × 2 mm slots are cut, 5 mm apart from the midline of the short wall, and 35 mm above the floor. In each slot, a plastic L-shaped pad, 15 × 5 cm and 1.5 mm thick, is inserted and used as a sliding lid to cover the vials that will contain the rewards (Figure 2). The last 20 mm of each pad's long side are folded upward to prevent their total extraction from the slots and to gently push away the mouse after the choice has been made.

Figure 2. Reward pedestal with sliding lids, top-front viewBetween the slots with the sliding lids, there is a pedestal carrying the stimuli (S+: reward; S-: no reward). The rewards are placed in two 15-ml Falcon tube lids positioned on the pedestal (see Figure 3). This pedestal is made of a small woodblock, 10 × 4 cm and 15 mm thick, divided into two equal halves by a transparent Plexiglas bar, 22 × 5 cm and 5 mm thick. Each half of the pedestal is lined with a pattern differing in color and texture and carries at its center, under the lining, a thin iron plate. The linings are made of white Teflon tape on one side and brown sandpaper on the other. Alternative linings, such as Velcro tape and PVC from floor tiles, can be used, but their salience equivalence should be checked in advance.
Materials the same as the lining are used to externally line the two lids of 15-ml Falcon tubes used as reward receptacles. A 2-mm magnet is glued under each lined vial to secure it in place on the pedestal.
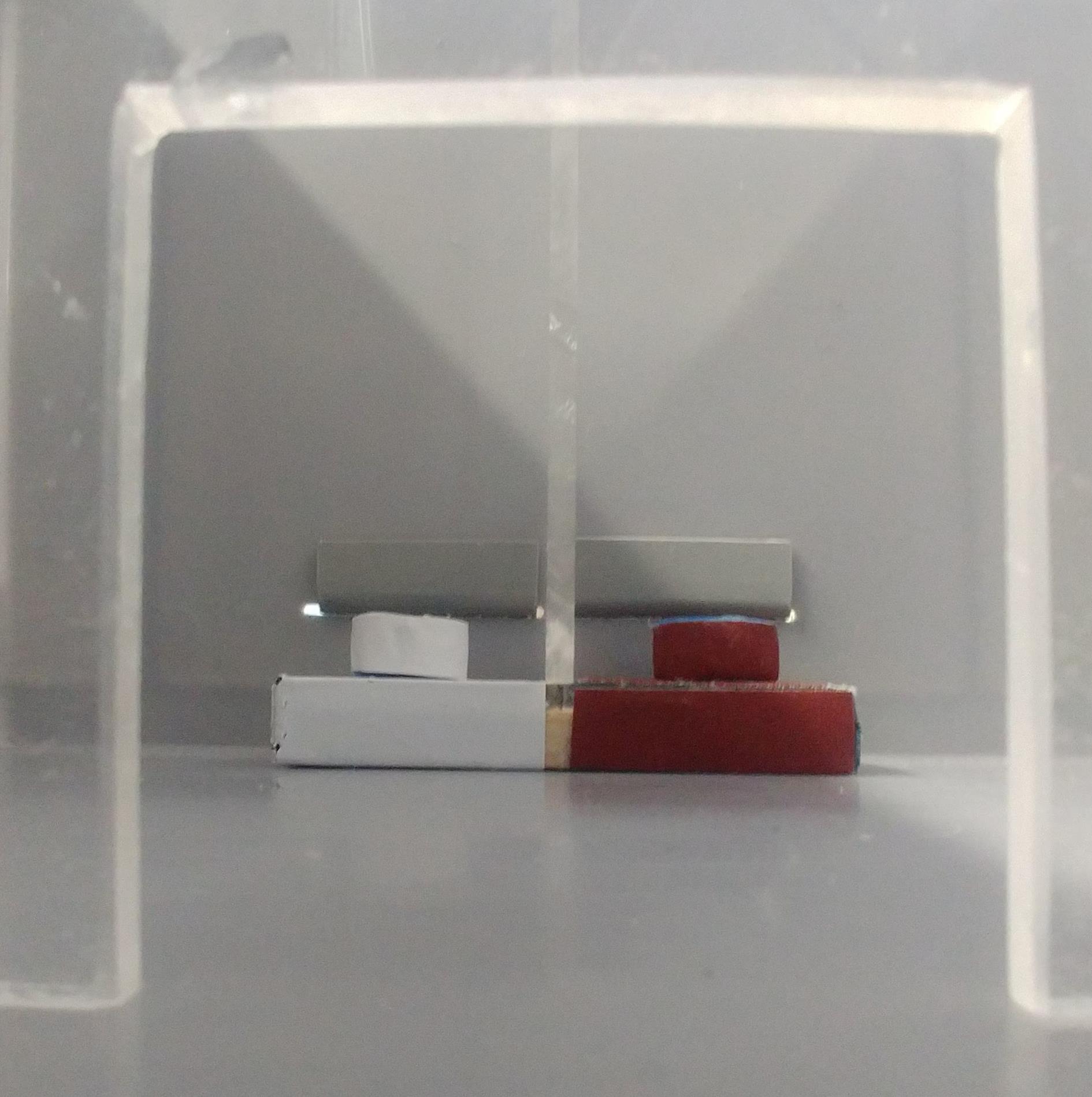
Figure 3. Reward pedestal from the frontDuring training and testing, the arena is placed on a table in a room with indirect illumination at 30-50 lux intensity. The experimenter should avoid bending over the arena while the mice are in the reward zone. Mice are observed via a monitor connected to a video camera.
Video camera and monitor
We used an Ikegami IR ICD47E analogic camera connected to a video monitor via a BMC cable. However, for the sake of monitoring the mice during the test, virtually any camera connected to a personal computer would work.
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Magara, F., Boury-Jamot, B. and Hörnberg, H. (2021). A Simple Spatial-independent Associative and Reversal Learning Task in Mice. Bio-protocol 11(15): e4108. DOI: 10.21769/BioProtoc.4108.
分类
神经科学 > 神经系统疾病 > 动物模型
神经科学 > 行为神经科学 > 学习和记忆
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link