- EN - English
- CN - 中文
Cell-free Translation: Preparation and Validation of Translation-competent Extracts from Saccharomyces cerevisiae
无细胞翻译:酿酒酵母翻译活性提取物的制备和验证
发布: 2021年09月20日第11卷第18期 DOI: 10.21769/BioProtoc.4093 浏览次数: 5147
评审: Xin XuKomuraiah MyakalaAnonymous reviewer(s)
Abstract
Cell-free translation is a powerful technique for in vitro protein synthesis. While cell-free translation platforms prepared from bacterial, plant, and mammalian cells are commercially available, yeast-based translation systems remain proprietary knowledge of individual labs. Here, we provide a detailed protocol for simple, fast, and cost-effective preparation of the translation-competent cell-free extract (CFE) from budding yeast. Our protocol streamlines steps combined from different procedures published over the last three decades and incorporates cryogenic lysis of yeast cells to produce a high yield of the translationally active material. We also describe techniques for the validation and troubleshooting of the quality and translational activity of the obtained yeast CFE.
Graphic abstract:
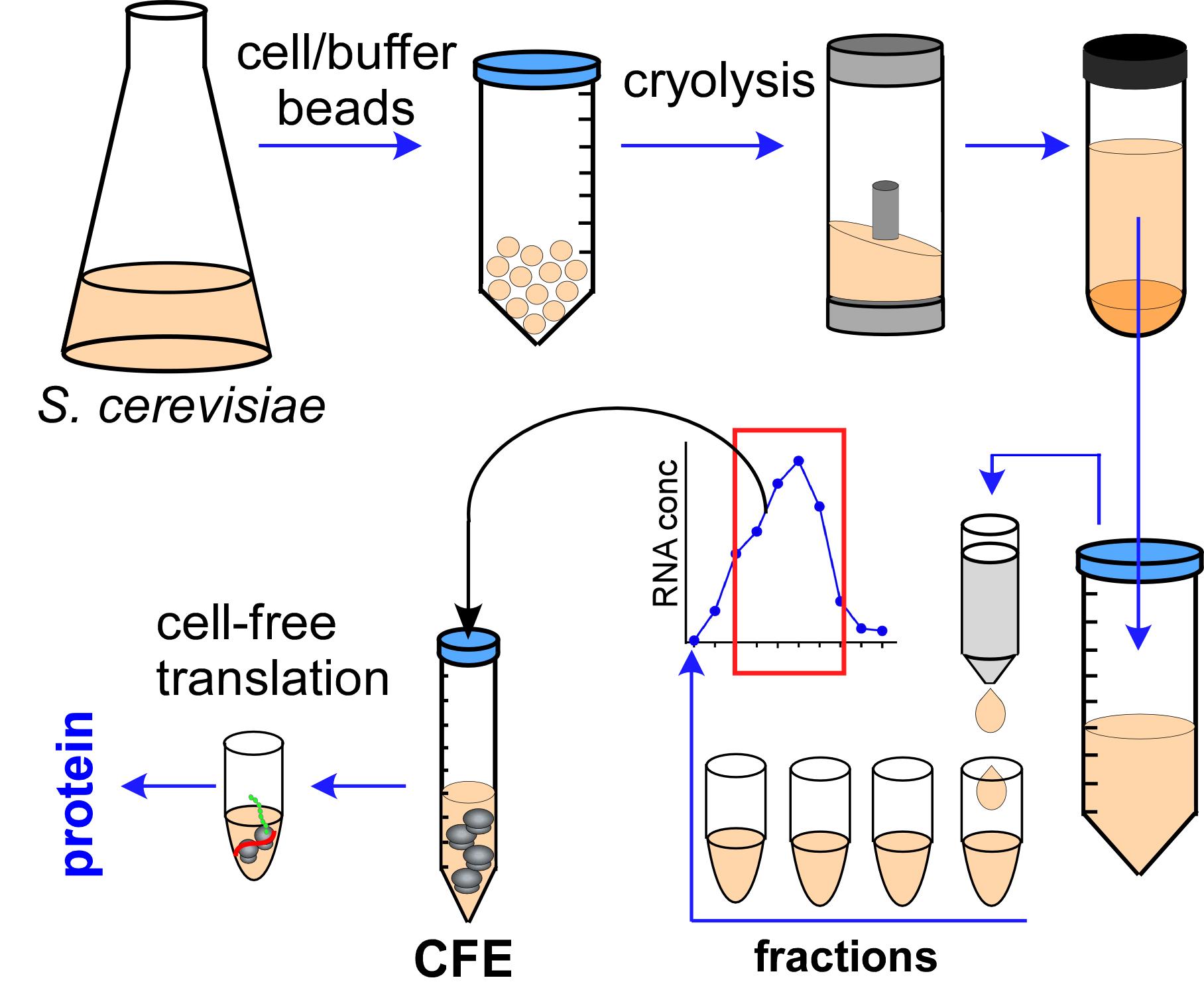
The flow of Cell-Free Extract (CFE) preparation procedure.
Background
Cell-free systems for protein synthesis make use of specially prepared cell extracts that retain macromolecular components required for translation, including ribosomes, tRNAs, aminoacyl tRNA synthetases, protein translation factors, and, in some cases, endogenous cellular mRNAs. The cell-free reactions can be charged with an mRNA of choice supplemented with radiolabeled or otherwise modified amino acids that become incorporated into nascent polypeptide chains during translation.
Cell-free translational platforms have been developed and optimized over the years for a plethora of organisms, such as E. coli (Shrestha et al., 2012; Kim et al., 2019), Bacillus subtilis (Kelwick et al., 2016), Pseudomonas putida (Wang et al., 2018), Streptomyces strains (Li et al., 2017), Leishmania trypanosomes (Kovtun et al., 2011), fungi Neurospora crassa (Wu et al., 2018), tobacco Bright Yellow 2 (BY-2) cells (Buntru et al., 2014), insect cells (Ezure et al., 2014), Chinese hamster ovary cells (CHO) (Brödel et al., 2014; Thoring and Kubick, 2018), and HeLa cells (Witherell, 2001; Mikami et al., 2010). For eukaryotic organisms, the most well-known and widely used cell-free translation systems have been those based on the rabbit reticulocyte (Pelham and Jackson, 1992; Olliver and Boyd, 1996) and wheat germ lysates (Harbers, 2014); both types are commercially available nowadays in several formulations. Besides generating both labeled and unlabeled proteins in a cell-free context, these reagents aided significantly in studying molecular mechanisms of protein synthesis, providing insights into individual steps of translation, translational efficiency, fidelity, and ribosome-associated protein quality control. In addition, cell-free translation systems have been instrumental in studies of protein evolution, enzyme engineering, high-throughput screens, and production of large quantities of proteins of interest for functional and structural analysis (reviewed in Carlson et al., 2012; Chong, 2014; Gregorio et al., 2019).
The budding yeast Saccharomyces cerevisiae represents an excellent source for translation-competent lysates. This unicellular eukaryotic organism combines the benefits of cost-effective microbial culture with an assortment of well-developed genetic tools: strains of interest can be easily generated with genes deleted (non-essential genes), depleted (essential genes), or overproduced. Moreover, a multitude of genetically modified yeast strains is already available from research laboratories and commercial sources. Despite many benefits, yeast-based translation extracts are not commercially available at present. Although several laboratory protocols have been published, many are too lengthy or incorporate cumbersome experimental manipulations, the rationale for which is not always clear. Guided by the published protocols, we developed a streamlined procedure, described below, that allows efficiently generating translation-competent S. cerevisiae extracts. Our main goal was to improve and simplify this technology to make it more user-friendly, reliable, reproducible, and affordable for any laboratory.
Unlike the previously documented lysis procedures that utilize enzymatic cell wall digestion and spheroplasting of yeast cells (Gasior et al., 1979; Tuite and Plesset, 1986) or the mechanical yeast cell lysis (Hodgman and Jewett, 2013; Hussain and Leibowitz, 1986; Schoborg et al., 2014; Tarun and Sachs, 1995; Tuite and Plesset, 1986; Tuite et al., 1980), we adapted a cryogenic lysis technique. This approach has several advantages over conventional lysis procedures: the translationally important components are protected from excessive degradation; precise amounts of cellular material can be taken for cryogenic grinding/milling and lysis, improving reproducibility between lysate preparations or among different strains. In addition, frozen yeast can be stored at -80°C either prior to or after cryogrinding for an extended time, creating breakpoints in the procedure. In our protocol, we adapted two consecutive centrifugation steps (at 30,000 × g and at 100,000 × g) as in procedures described by Hofbauer et al. (1982) and Tuite and Plesset (1986). These steps allow separation of the translationally active cellular fraction from heavy particles, lipids and polysaccharides. After a gel filtration step on Sephadex G-25 columns, we use a glycerol-containing buffer, which in our hands results in longer storage of an active lysate. Unlike many published protocols (Hodgman and Jewett, 2013; Hussain and Leibowitz, 1986; Schoborg et al., 2014; Tarun and Sachs, 1995; Tuite and Plesset, 1986), we omitted treatment of the cell-free extract (CFE) with micrococcal nuclease, designed to remove the endogenous mRNA. Although the cellular mRNA might interfere with translation of the reporter, we find that for many types of experiments, this step is unnecessary, and it also tends to lead to high variability between different CFE batches.
Here, we provide a detailed protocol for preparing translationally competent lysates from budding yeast, which has been applied in our work to study unconventional translation initiation mechanisms driven by 5’-UTRs (Trainor et al., 2021).
Materials and Reagents
PD-10 columns Sephadex G-25 (20 × 80 mm) (GE Healthcare, catalog number: 17085101); store at room temperature
15 ml conical centrifuge tubes (RNase-free) (VWR, catalog number: 10026-076) or equivalent
50 ml conical centrifuge tubes (RNase-free) (VWR, catalog number: 10026-078) or equivalent
1.7 ml microcentrifuge tubes (RNase-free) (Denville, catalog number: C2170) or equivalent
PCR tubes (USA Scientific, catalog number: 1402-2900) or equivalent
Ultracentrifuge tubes (Beckman Coulter, model: 10.4 ml polycarbonate bottles with cap assembly (16 × 76 mm) for the Ti80 rotor; catalog number: 355603)
Volumetric Serological pipettes, RNase-free, for 25 ml (Denville Scientific Inc, catalog number: P7129) and for 5 ml (Denville Scientific Inc, catalog number: P7127) or equivalent
25 mm Syringe filter (Corning, catalog number: 431224)
Rapid flow bottle top PES filter, 500 ml (Thermo Scientific, catalog number: 73520-994)
Sterile Pasteur pipettes (Thomas Scientific, SteriPettes, catalog number: 1215D97) or equivalent
BY4741 strain (Fisher Scientific; Dharmacon Inc, YEAST PARENT STRAIN BY4741, YSC1048); store in YPD/20% glycerol stock at -80°C
Peptone (Research Products International, catalog number: P20250); store at room temperature
Yeast extract (Thermo Scientific, catalog number: J23547-A1); store at room temperature
Dextrose (VWR, BDH Chemicals, catalog number: BDH9230); store at room temperature
Bacto-agar (VWR, Life Sciences, catalog number: J637); store at room temperature
RNase away solution (Thomas Scientific, catalog number: 1236B62); store at room temperature
HEPES free acid (VWR Life Sciences, catalog number: 0511); store at room temperature
Potassium acetate, C2H3O2K (Bio Basic, catalog number: PB0438); store at room temperature
Magnesium acetate tetrahydrate, C4H6MgO4·4H2O (Sigma, catalog number: M5661); store at room temperature
Mannitol powder (VWR BDH Chemicals, catalog number: BDH9248); store at room temperature
Dithiothreitol (DTT) (Sigma, catalog number: D0632); store at 4°C
Renilla luciferase plasmid, pRL-null (Promega, catalog number: E2271); store at -20°C
Firefly plasmid, pGEM-luc (Promega, catalog number: E154A); store at -20°C
Nano luciferase plasmid, pF4Ag NanoLuc (Addgene, catalog number: 137777); store at -20°C
DNA clean & concentrator kit (ZYMO RESEARCH, catalog number: D4004); store at room temperature
mMESSAGE mMACHINETM T7 Transcription kit (Thermo Fisher Scientific, Invitrogen, catalog number: AM1344); store at -20°C
Creatine kinase, rabbit muscle (BioVision, catalog number: P1301); store at -20°C
Creatine phosphate (VWR, catalog number: 97061-328); store at -20°C
GTP solution, 100 mM (Thermo Fisher Scientific, catalog number: R0461); store at -20°C
RiboLock RNase inhibitor, 40 U/μl (ThermoFisher Scientific, catalog number: EO0381); store at -20°C
Renilla Luciferase Assay System (Promega, catalog number: E2810); store at -20°C
Firefly Luciferase Assay Systems (Promega, catalog numbers: E1500, E4030, E4550) store at -20°C
Nano-Glo Luciferase Assay Systems (Promega, catalog numbers: N1110, N1120) store at -20°C
Renilla luciferase monoclonal antibody (Thermo Fisher Scientific, catalog number: SR07-830), store at 4°C
TAP polyclonal antibody (Thermo Fisher Scientific, catalog number: CAB1001), store at 4°C
Rpl3 monoclonal antibody (ScRPL3, Developmental Studies Hybridoma Bank, University of Iowa), store at 4°C
EasyTag EXPRESS35S Protein Labeling mix, [35S]-, 2 mCi, 11 mCi/ml (PerkinElmer, catalog number: NEG772002MC), store at 4°C
Trichloroacetic acid, TCA (Sigma, catalog number: T6399); store at 4°C
UltraPure Agarose (Invitrogen, catalog number: 16500); store at room temperature
Formamide (Sigma, catalog number: 47670-25ML-F); store in 1 ml aliquots at -80°C
SYBR gold nucleic acid gel stain (Thermo Fisher Scientific, Invitrogen, catalog number: S11494); store at -20°C
Optional: Hybond-N+ Nylon membrane (GE Healthcare, catalog number: NS0921); store at room temperature
Optional: TRI-REAGENT-LS (Molecular Research Center Inc., catalog number: TS 120); store at 4°C
HCl (GFS Chemicals, catalog number: 43491); store at room temperature
Glycerol (VWR Life Sciences, catalog number: BDH1172); store at room temperature
Liquid nitrogen
DreamTaq PCR master mix (2×) (ThermoFisher Scientific, catalog number: K1071); store at -20°C
GeneRuler DNA ladder mix (Thermo Fisher Scientific, catalog number: SM0333); short term storage 4°C, long term storage -20°C
ATP solution, 100 mM (Thermo Fisher Scientific, catalog number: R0441); store at -20°C
1 mM solution of 20 Essential amino acids, complete (Promega, catalog number: L4461); store at -20°C
1 mM solution of Essential amino acids minus Methionine and Cysteine (Promega, catalog number: L5511); store at -20°C
Formaldehyde solution, 37% (GFS Chemicals, catalog number: 40301); store at room temperature
EDTA, 0.5 M sterile solution, RNase-free (VWR Life Sciences, catalog number: E522-100ML); store at room temperature
Yeast medium (see Recipes)
YPD medium
YPD-agar plates
Stock solutions (see Recipes)
1 M HEPES-KOH, pH 7.6
2 M KOAc
300 mM MgOAc
100 mM MgOAc
1 M DTT
50% glycerol
Working solutions (see Recipes)
Buffer A
Buffer A/glycerol
Buffer A/mannitol
Buffer A/mannitol/DTT
Buffer A/glycerol/DTT
Creatine kinase dilution buffer
Creatine kinase solution
10× Energy mix
FAE solution
Equipment
-80°C freezer
-20°C freezer
Lab water purification system (PureLab, model: Elga water polisher system) or equivalent
pH meter (Sartotius, model: PB-11-P11.1) or equivalent
Optional: Laminar Flow Hood (NuAir, NU-201-430)
Microbiological incubator (Binder World, Series ED) or equivalent
Incubator shaker (Eppendorf, model: New Brunswick Innova® 43) or equivalent temperature-controlled shaker that can fit a 4 L flask
Preparative centrifuge (Beckman Coulter, model: J2-MI) or equivalent
Beckman JLA-10.500 rotor (Beckman Coulter) or equivalent
Preparative ultracentrifuge (Beckman Coulter, model: Le-80)
Beckman ultracentrifuge rotor (Beckman Coulter, model: Ti80)
Eppendorf centrifuge with the A-4-62 rotor, refrigerated (Eppendorf, model: 5810R)
Benchtop centrifuge (Eppendorf, model: 5420)
Freezer/Mill® (SPEX SamplePrep, model: 6700) or equivalent Cryogenic grinder
Freezer/Mill® accessories: small grinding vial set (SPEX SamplePrep, catalog number: 6751)
Toplanding balance (Sartorius, catalog number: CP3202P) or equivalent
Pipetman (Gilson, P20, P200, and P1000) or equivalent
Vortexer (any model)
Spectrophotometer (Eppendorf, catalog number: 6131-26951) or equivalent
500 ml Erlenmeyer flask
4 L Erlenmeyer flask
PPCO centrifuge bottles with sealing closure, 500 ml (Thomas Scientific, catalog number: 2625H84) or equivalent
Thermocycler (Eppendorf, model: Mastercycler®) or equivalent
Dry bath incubator (Eppendorf, model: Thermomixer R)
Luminometer (Promega; model: Glomax 20/20) or equivalent
Liquid scintillation counter (Beckman Ls System, model: Ls6000Ta) or equivalent
Power supply (Bio-Rad, model: PowerPac Universal Power Supply, catalog number: 1645070) or equivalent
Agarose gel electrophoresis equipment (Mansour and Pestov, 2013)
Gel rocker platform (Thomas Scientific, BioRockerTM 3D Mini Rockers, catalog number: 1155J96) or equivalent
Typhoon imager (GE, model: Typhoon 5 Biomolecular Imager), or any gel-imaging system (for example, Cole-Parmer, model: DelLogic 100 imaging system, or equivalent)
Protein gel electrophoresis equipment (Bio-Rad, model: Mini-PROTEAN tetra Vertical Electrophoresis Cell, catalog number: 1658004) or equivalent
Optional: polyacrylamide gel electrophoresis equipment (Hoefer Inc, model: SE260 Mighty Small II Delux Mini Vertical electrophoresis Unit)
Optional: Transfer Cell (Bio-Rad, Trans-Blot® SD Semi-Dry Transfer Cell, catalog number: 1703940) or equivalent
Optional: Hybridization oven (UVP, model: HB-1000 Hybridizer) or equivalent
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Trainor, B. M., Komar, A. A., Pestov, D. G. and Shcherbik, N. (2021). Cell-free Translation: Preparation and Validation of Translation-competent Extracts from Saccharomyces cerevisiae. Bio-protocol 11(18): e4093. DOI: 10.21769/BioProtoc.4093.
分类
微生物学 > 微生物遗传学 > RNA
生物化学 > RNA > mRNA翻译
生物化学 > 蛋白质 > 表达
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













