- EN - English
- CN - 中文
Method for Rapid Enzymatic Cleaning for Reuse of Patch Clamp Pipettes: Increasing Throughput by Eliminating Manual Pipette Replacement between Patch Clamp Attempts
膜片钳移液管重复使用的快速酶清洗方法:通过消除膜片钳尝试之间的手动移液管更换来提高吞吐量
发布: 2021年07月20日第11卷第14期 DOI: 10.21769/BioProtoc.4085 浏览次数: 4445
评审: Alexandros C KokotosAkira KarasawaCarmelo Bellardita
Abstract
The whole-cell patch-clamp method is a gold standard for single-cell analysis of electrical activity, cellular morphology, and gene expression. Prior to our discovery that patch-clamp pipettes could be cleaned and reused, experimental throughput and automation were limited by the need to replace pipettes manually after each experiment. This article presents an optimized protocol for pipette cleaning, which enables it to be performed quickly (< 30 s), resulting in a high yield of whole-cell recording success rate (> 90%) for over 100 reuses of a single pipette. For most patch-clamp experiments (< 30 whole-cell recordings per day), this method enables a single pipette to be used for an entire day of experiments. In addition, we describe easily implementable hardware and software as well as troubleshooting tips to help other labs implement this method in their own experiments. Pipette cleaning enables patch-clamp experiments to be performed with higher throughput, whether manually or in an automated fashion, by eliminating the tedious and skillful task of replacing pipettes. From our experience with numerous electrophysiology laboratories, pipette cleaning can be integrated into existing patch-clamp setups in approximately one day using the hardware and software described in this article.
Graphic abstract:
Rapid enzymatic cleaning for reuse of patch-clamp pipettes
Background
Whole-cell patch-clamp recordings allow unprecedented access to electrical activity, neuronal morphology, and gene expression at the single-cell level (Jiang et al., 2015; Gouwens et al., 2019). However, because this method requires a great amount of skill and care to perform correctly, it remains one of the most difficult in neuroscience. A crucial step in this method is the formation of a tight, high resistance (e.g., >1 GΩ) connection between the cell membrane and the glass pipette known as a gigaseal. Gigaseal formation requires a clean pipette surface, and even small contaminants (e.g., cell debris or dust) can disrupt this process (Hamill et al., 1981). For this reason, patch-clamp experimenters need to replace glass pipettes after each recording attempt (Figure 1A), requiring additional time and attention (e.g., removal, fabrication, filling, and installation of pipettes). We have previously found that contrary to decades of this ubiquitous practice in the field, patch-clamp pipettes can be cleaned and reused, enabling many patch clamp recordings with a single pipette (Kolb et al., 2016).
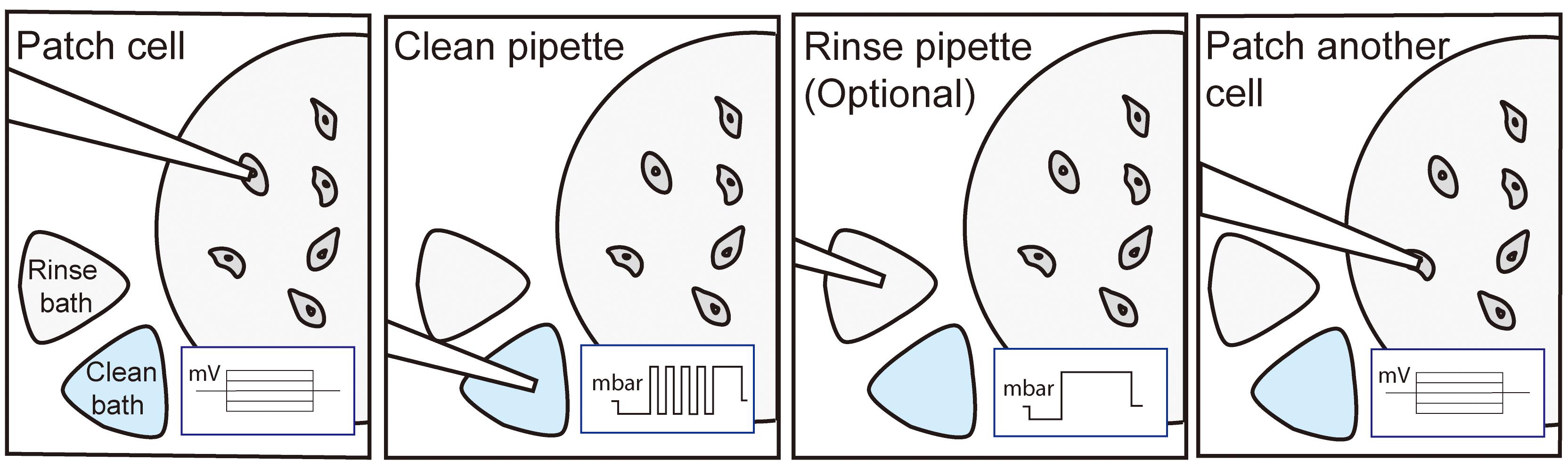
Pipette cleaning is a robust, simple process involving the following steps: (1) attempt whole-cell patch-clamp recording; (2) retract patch-clamp pipette and move towards bath containing cleaning solution; (3) with tip submerged in cleaning solution, cycle positive and negative pressures to remove cell debris from pipette tip; (4) position pipette over new target cell for second patch-clamp attempt; and (5) repeat steps 1-4 until the experiment is completed or the pipette fails (e.g., tip breakage, clog, evaporation of cleaning solution, or user error). In this protocol, we will describe the use of simple hardware and software to automate pipette cleaning, which will assist other labs in implementing pipette cleaning in either fully automated or “push-to-clean” methods (Figure 1B).

Figure 1. Pipette cleaning methods. A. Process flow chart for traditional manual patch clamping without pipette cleaning. Removing, filling, and installing fresh pipettes takes between 60-120 s. B. Process flow chart for manual patching with automated cleaning (“push-to-clean”). Automated cleaning can be run in as little as 30 s. C. Close up images of pipette being moved from the experimental chamber (left) to the cleaning bath (middle) and to the rinse bath (right) before returning to the experimental bath to patch another cell. Scale bar is 25 mm. D. Custom experimental chamber for pipette cleaning featuring fluid inlet and outlet, inset for ground wire, and external baths for cleaning and rinsing solutions. Scale bar is 1 cm.
We published our initial discovery of this method in 2016 and used it to develop the first fully autonomous patch-clamp robot capable of recording dozens of cells with no human supervision in 2019 (Kolb et al., 2016 and 2019). This pipette cleaning method has been used by us, our collaborators, and other groups to make large-scale patch-clamp studies (i.e., single-cell electrophysiology and connectomics in rodents and humans and high throughput screening) more efficient (Peng et al., 2019; Koos et al., 2020) and to make complex experiments (i.e., in vivo patch-clamp) simpler (Suk et al., 2017; Stoy et al., 2020). In addition, since our initial report, we have discovered that 2% w/v Tergazyme is a superior cleaning solution (Figure 2A) and that the rinsing step from the original method is unnecessary (Figure 5). These improvements to the method have increased the whole-cell recording yield ~20%, increased the number of total cleans with a single pipette by a factor of 10 in HEK 293 cells (Figure 4), and decreased the time needed for each round of cleaning to <30 s, faster than manual pipette replacement (~1-2 min). Building on these gains in efficiency, we improved whole-cell yield to ~90% in HEK 293 cells by optimizing the position of the pipette tip relative to the cell membrane (Figure 2A-C) (Stoy et al., 2020). Briefly, by varying the distance the pipette was advanced into the cell, we found a strong relationship between distance and gigaseal probability, which reached ~100% at a range of 1-2 µm below the cell surface (defined as the z-axis point where pipette resistance increased 0.1 MΩ from initial resistance) (Stoy et al., 2020). When the patcherBot was programmed to attempt gigasealing at this position, the whole-cell recording yield increased significantly, as shown in the “Optimized” trace of Figure 2A (P = 0.044, Kolmorogov-Smirnov test). This method is used by the patcherBot in Figure 3 and Figure 4. Overall, this improved pipette cleaning method enables automated patch-clamp experiments to operate completely unattended for 3-4 h at yields of up to 90% and throughputs of up to 15 whole-cell recordings per hour, surpassing the output of highly skilled human experimenters (Figure 2, Figure 3). We were able to achieve up to 100 whole-cell recordings in ~13 h with a single pipette (Figure 4). We have also developed a simple hardware and software package for “push-to-clean” semi-automated patch-clamp experiments, enabling electrophysiologists to easily integrate this method into their hardware setup (Figure 1B-D, Figure 6, and Supplemental Information).
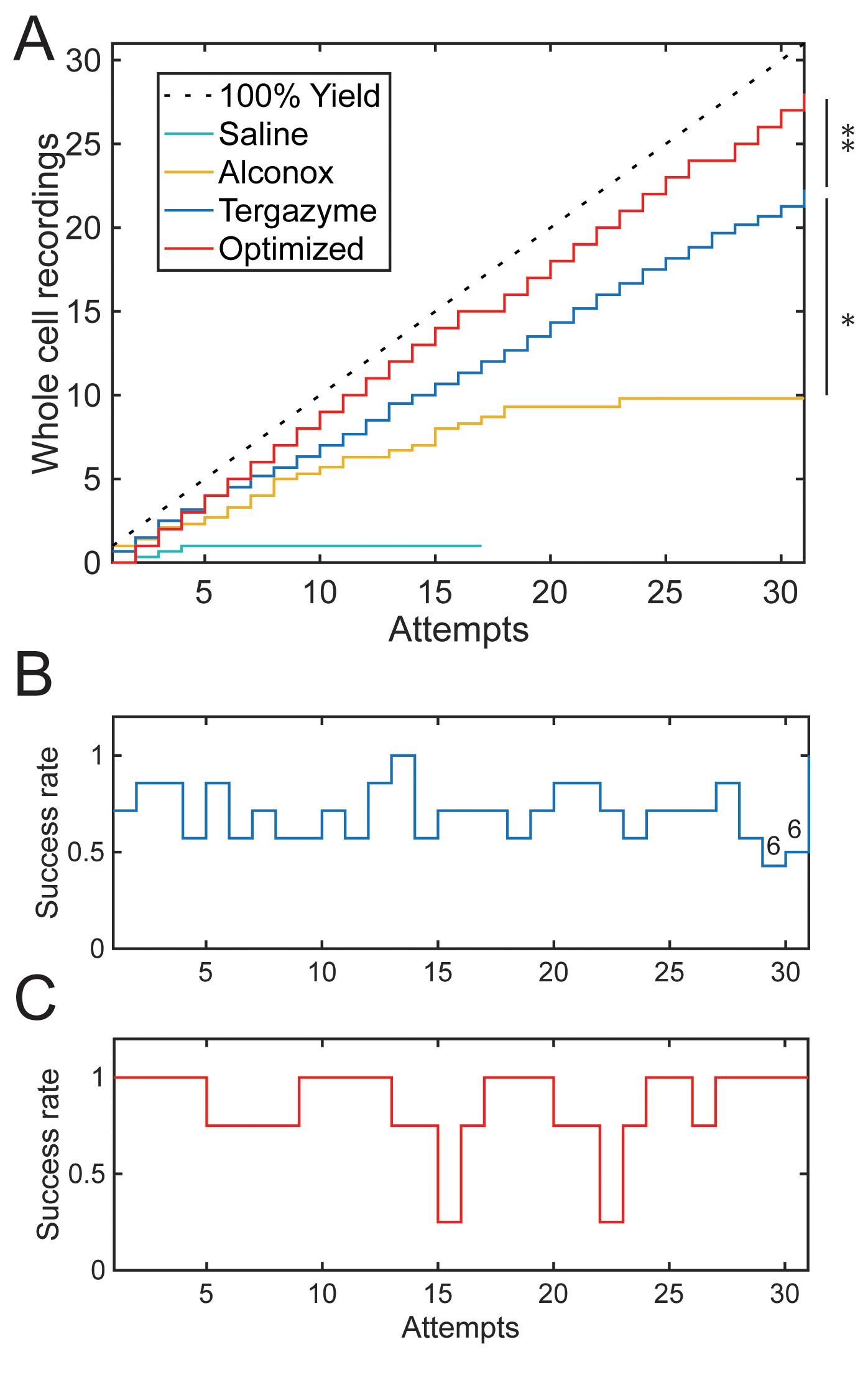
Figure 2. Improvements to pipette cleaning. A. Each trace represents the number of whole-cell recordings in HEK 293 cells as a function of the number of recording attempts with a single pipette. Each trace is the average of at least three pipettes. The “Saline” trace is a negative control (i.e., cleaning solution replaced with extracellular solution), and a 100% theoretical maximum is included for reference. The “Alconox” trace shows the performance of 2% w/v Alconox cleaning, which decreases as a function of the number of attempts. The “Tergazyme” trace shows no decrease in yield for 30 attempts with 2% w/v Tergazyme. The “Optimized” trace represents 2% w/v Tergazyme cleaning with optimized pipette positioning relative to the cell surface for gigasealing. The “Tergazyme” performance is superior to that of Alconox (*, P = 1.375E-5, Kolmogorov-Smirnov test). The “Optimized” performance is superior to that of “Tergazyme” (**, P = 0.04368, Kolmogorov-Smirnov test). B. Success rate of whole-cell patch-clamp as a function of the number of cleans using 2% w/v Tergazyme shows no significant decrease in likelihood of subsequent whole-cell recording (Odds ratio (OR) = 1.0067, CI: 0.97-1.04, P = 0.69, n = 215 attempts, each attempt is for n = 7 pipettes, except attempts 29 and 30, which are for n = 6). C. Optimized indentation with Tergazyme cleaning shows no significant decrease in likelihood of subsequent whole-cell recording (OR = 1.00, CI: 0.94-1.06, P = 0.95, n = 124 attempts, each attempt is for n = 4 pipettes).
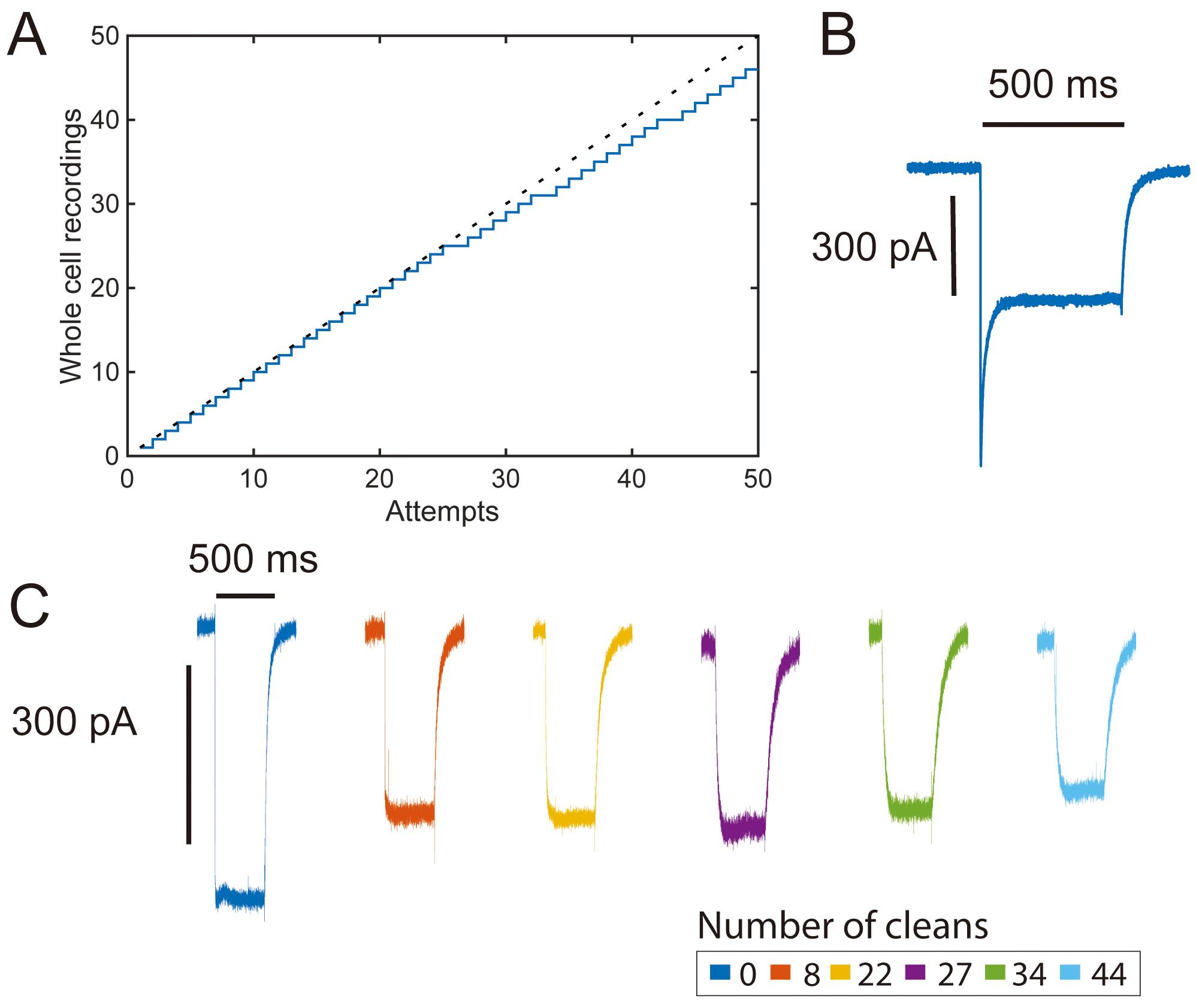
Figure 3. High-throughput opsin screening with pipette cleaning. A. Yield curve for a single pipette channelrhodopsin-2 (ChR-2) characterization experiment (46/51 attempts, 90% yield). B. Representative photocurrent trace (voltage clamp) in response to an initial pulse of 500 ms 480 nm LED pulse recorded from transiently transfected HEK 293 cell showing a large peak photocurrent response. C. Photocurrent traces (voltage clamp) from cells recorded in the middle of a series of 500 ms light pulses showing steady-state photocurrents over many pipette cleans. Large initial photocurrent responses are typical of ChR-2 (B) and are reduced in subsequent stimulation pulses (C) (Lin et al., 2009).
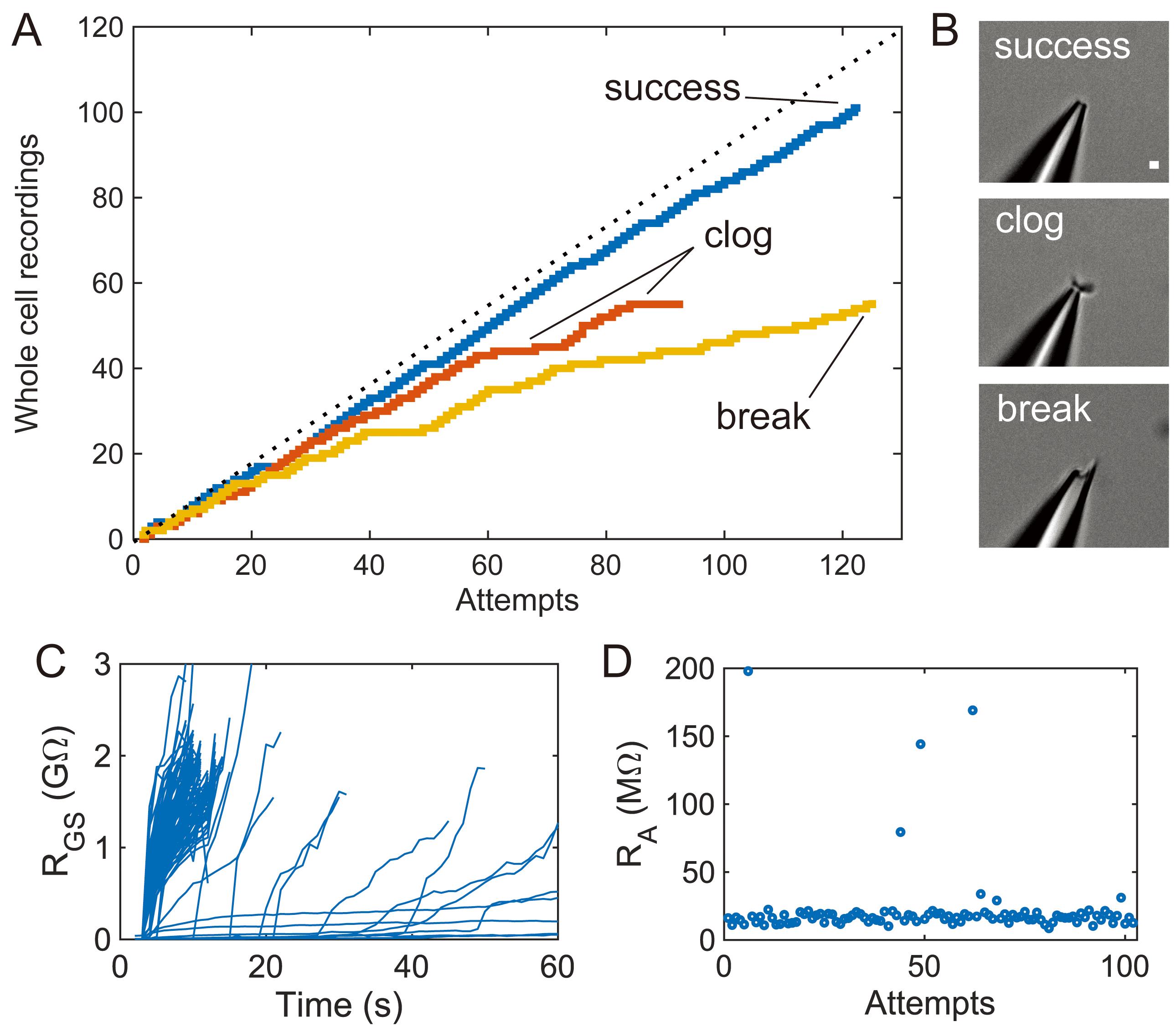
Figure 4. Upper limits of pipette cleaning with 2% w/v Tergazyme. A. Yield curves for individual pipettes showing cleaning for over 90 recording attempts with associated failure modes. The “success” trace shows effective pipette cleaning, the “clog” trace shows reversible pipette tip clogs that cause low yield over time, and the “break” trace shows experiments terminated by broken pipette tips. Theoretical maximum (100% yield) included for reference. B. Representative pipette images taken at 40× magnification for each failure mode in (A). Scale bar is 1 µm. C. Individual gigaseal resistance traces from the “success” trace (n = 122 gigaseal attempts). D. Access resistance of cells recorded in the “success” trace (n = 101 whole-cell recordings).
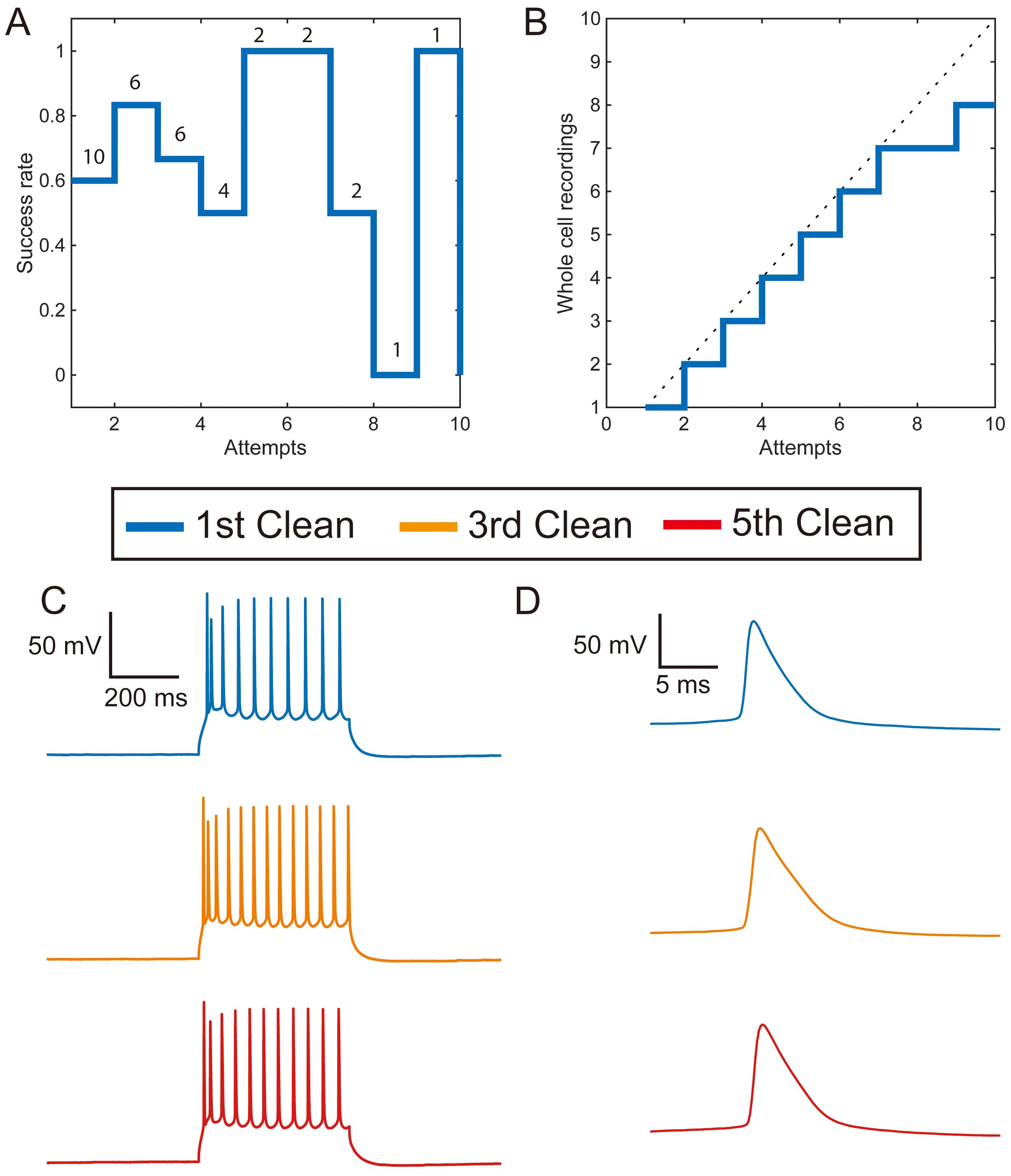
Figure 5. Tergazyme cleaning is effective without a rinsing step in acute mouse brain slices. A. The success rate of patching without a rinse step does not decrease significantly with the number of cleans (OR = 1.14, CI: 0.87-1.41, P = 0.34, n = 36 attempts). The number of pipettes used for each experiment is noted above each number of reuses. B. Yield plot for a single pipette using 2% w/v Tergazyme without rinsing. C. Representative recordings of evoked action potential firing in current clamp from three neurons recorded with a single pipette cleaned in 2% w/v Tergazyme without rinsing. D. Enlarged single evoked action potentials from the neurons in (C).
A robust and easy-to-implement method for automated pipette cleaning is of interest to all laboratories using the patch-clamp method. Although no negative side effects have been measured in cleaning experiments to date (Kolb et al., 2016 and 2019; Peng et al., 2019), for experiments where the possibility of exposing sensitive cells to cleaning residues is of particular concern (e.g., pharmacology or single-channel recordings), the method can be easily adapted to further minimize this risk (see Notes). We believe that this improved pipette cleaning method will be especially useful to labs working in the areas of multi-pipette patch clamping and high-throughput screening (see Notes, Figure 3).
The pipette cleaning method described in this protocol enables pipette reuse for patch clamping. By eliminating the need to fabricate, fill, install, and remove pipettes throughout experiments, experimenters can save valuable time and attention from these labor-intensive tasks. We show that 2% w/v Tergazyme enables up to and over 100 cells to be recorded with a single pipette (5-8 MΩ, Warner Instruments), eliminating the need for experimenters to replace pipettes over the course of an experimental day.
Materials and reagents
Borosilicate pipette glass with filament (Warner Instruments, catalog number: 64-0793)
Syringe, 5 ml (VWR, catalog number: BD309646)
Syringe filter, 0.2 µm (VWR, catalog number: 10218-486)
23G needle (VWR, catalog number: 89134-098)
Tergazyme (Alconox, catalog number: 1304-1)
Equipment
The equipment listed is in addition to standard patch-clamp electrophysiology equipment (e.g., amplifier, digitizer, headstage, micromanipulator, microscope, and pipette puller). Specific details of the patch-clamp rig used in this paper are described in detail elsewhere (Kolb et al., 2016 and 2019).
Cleaning dish (3D print or mill according to CAD files in SI, with appropriate changes for microscope stage)
Pressure control box (detailed plans and parts list on autopatcher.org, direct order from Neuromatic Devices, neuromaticdevices.com)
Software
Depending on the level of automation desired, download either (1) and (2) for full automation or only (2) to enable “push-to-clean” for manual patch clamping with cleaning.
Autopatcher software (downloadable at autopatcher.org). This software enables full automation of the cell detection, gigasealing, and break-in functions.
Push-to-clean software (downloadable at Github, https://github.com/mightenyip/Pipette-Cleaning-Software). The terminology “push-to-clean” is defined as an otherwise manual electrophysiology rig that includes a button-actuated pipette cleaning function. The button initiates a series of pipette position and pressure changes to clean the pipette for reuse.
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Landry, C. R., Yip, M. C., Kolb, I., Stoy, W. M., Gonzalez, M. M. and Forest, C. R. (2021). Method for Rapid Enzymatic Cleaning for Reuse of Patch Clamp Pipettes: Increasing Throughput by Eliminating Manual Pipette Replacement between Patch Clamp Attempts. Bio-protocol 11(14): e4085. DOI: 10.21769/BioProtoc.4085.
分类
神经科学 > 基础技术 > 快速制片
生物物理学 > 电生理 > 膜片钳技术
生物科学 > 生物技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












